Know the Science: How Medications and Supplements Can Interact
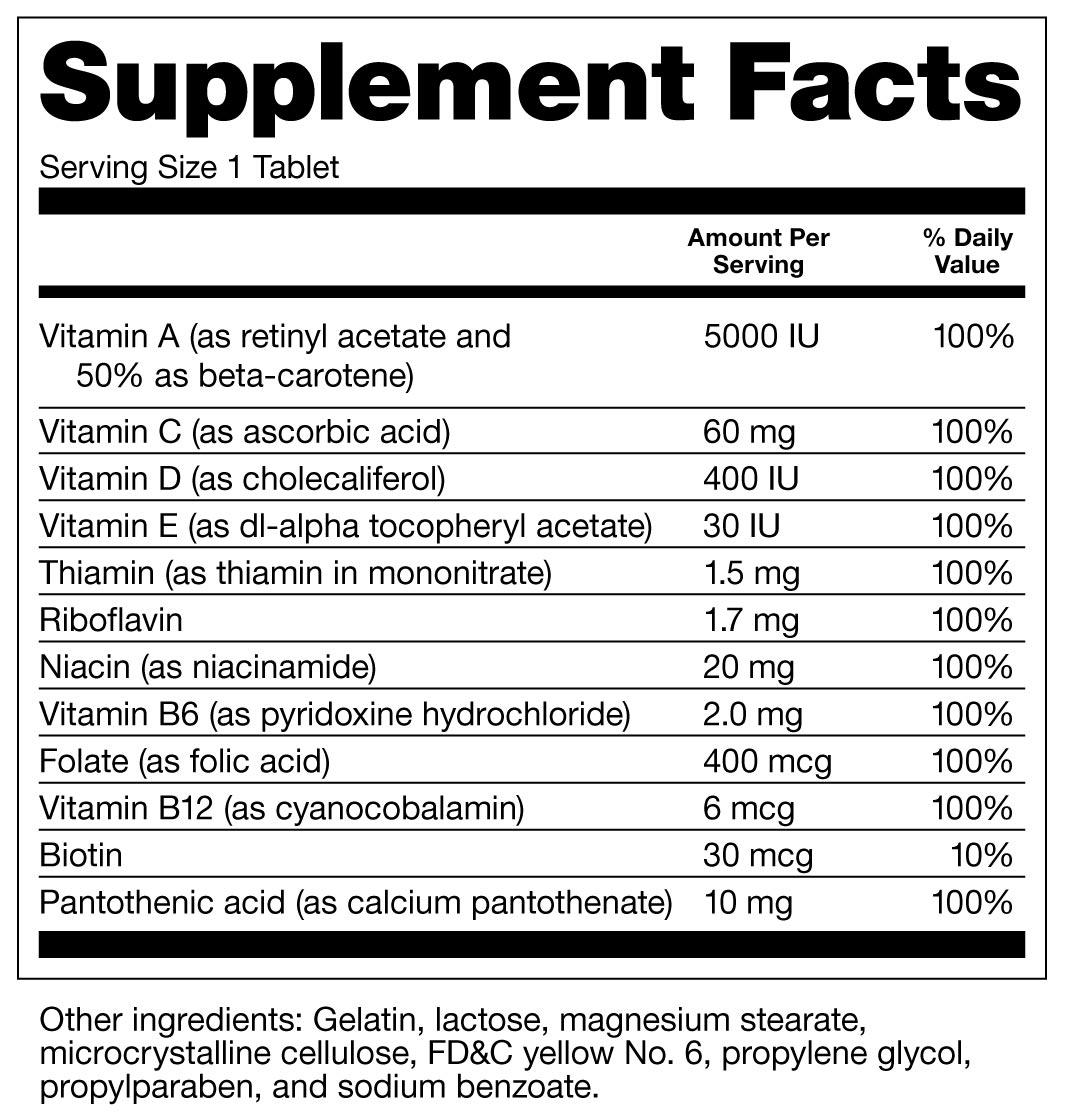
Tips on Reading Supplement Labels
Sometimes it isn’t obvious what’s in the bottle of a dietary supplement.
To find out what’s in a supplement, look for the Supplement Facts panel on the product label. The manufacturer is required to list all the supplement’s ingredients on this panel, either in the Supplement Facts chart or in the list of other ingredients below it.
It’s important to know the U.S. Food and Drug Administration has the authority to act against any adulterated, mislabeled dietary supplement product after it reaches the market. In addition, products with labels indicating they have been tested by independent, third-party groups such as nonprofit U.S. Pharmacopoeia (USP) Dietary Supplement Verification Program or ConsumerLab.com is a good way to ensure that a dietary supplement contains what’s on the label, is of high quality, and is not contaminated or adulterated with other materials.