Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Programs
NCCIH’s Small Business Program
The National Center for Complementary and Integrative Health (NCCIH) offers two distinct funding mechanisms that advance small businesses:
- The Small Business Innovation Research (SBIR) program
- The Small Business Technology Transfer (STTR) program
See More
Together, these programs are known as America’s Seed Fund. The SBIR and STTR programs are one of the largest sources of early-stage capital for technology commercialization in the United States. Both programs allow U.S.-owned and -operated small businesses to engage in Federal research and development that has a strong potential for commercialization. The National Institutes of Health’s (NIH) SBIR and STTR programs invest over 1 billion dollars each year into health and life science companies that are creating innovative technologies that align with NIH’s mission to improve health and save lives. A key objective is to translate promising technologies to the private sector and enable lifesaving innovations to reach consumer markets.
We invite you to explore the NCCIH strategic plan to learn more about how your idea fits with NCCIH’s mission and priorities. Please also view a list of active funding announcements. Businesses interested in exploring SBIR/STTR grant opportunities with us are encouraged to contact the NCCIH small business team prior to submitting an application.
You can search for examples of projects currently funded by NCCIH by using NIH RePORTER.
Understanding the SBIR/ STTR Programs
Both the SBIR and STTR programs are divided into the three phases listed below. NIH has special Technical Assistance Programs to help small businesses move their technologies from the lab into the hands of customers. The NIH Niche Assessment Program and the I-Corps at NIH program are for Phase I awardees, and the NIH Commercialization Accelerator Program is for Phase II or Phase IIB awardees.
Phase I: Feasibility and Proof of Concept. The objective of Phase I is to establish the technical merit, feasibility, and commercial potential of the proposed research/research and development (R/R&D) efforts and to determine the quality of performance of the small business awardee organization prior to providing further Federal support in Phase II. Click on “See More” below to view NCCIH-Specific Budget and Duration Policies.
Phase II: Research/Research and Development. The objective of Phase II is to continue the R/R&D efforts initiated in Phase I. Funding is based on the results achieved in Phase I and the scientific and technical merit and commercial potential of the project proposed in Phase II. Only Phase I awardees are eligible for a Phase II award. Click on “See More” below to view NCCIH-Specific Budget and Duration Policies.
Phase III: Commercialization. The objective of Phase III, where appropriate, is for the small business to pursue commercialization objectives resulting from the Phase I/II R/R&D activities. The NIH SBIR/STTR programs do not fund Phase III, and NIH does not generally provide any Phase III funding to small businesses.
See More
NIH also has a Fast-Track application that allows small businesses to submit one application for Phase I and Phase II, a Direct SBIR Phase II solicitation that permits small businesses to bypass a Phase I award if they have already proved the feasibility of their technology, and a Commercialization Readiness Pilot Program solicitation that can help support commercialization activities. For more information about which solicitation is best suited for your small business, view the Funding page and speak to the appropriate SBIR/STTR program manager.
NCCIH Participation in Fast-Track: NCCIH does not participate in the Fast-Track option through NCCIH-issued SBIR/STTR Funding Opportunity Announcements (FOAs). If interested in pursuing a Fast-Track option via the Omnibus solicitations, please contact a member of the NCCIH SBIR/STTR team.
As listed in NOT-AT-20-017, “NCCIH Policy Change to Budget Limit and Grant Durations for SBIR/STTR Phase I and Phase II Applications,” for budgetary, administrative or programmatic reasons, NCCIH may decide not to fund an application or may decrease the length of an award and/or the budget recommended by a review committee.
Through this Notice, NCCIH has revised its budget and duration policies for SBIR/STTR Phase I and Phase II applications. Generally, NCCIH will not fund:
- Phase I applications greater than $256,580 total costs for the duration of the project or project periods greater than 2 years.
- Phase II applications greater than $1,710,531 total costs for the duration of the project or project periods greater than 3 years.
For “National Institutes of Health SBA-Approved SBIR/STTR Topics for Awards Over Statutory Budget Limitations,” the Small Business Administration (SBA) has approved an NIH SBIR/STTR Topic Waiver list for which NCCIH generally will not fund:
- Phase I applications greater than $325,000 total costs per year or project periods greater than 2 years.
- Phase II applications greater than $2,000,000 total costs for the duration of the project or project periods greater than 3 years.
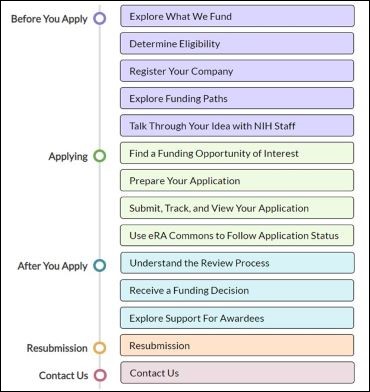
How To Apply: Step-By-Step Instructions
This interactive infographic from the NIH SEED website provides step-by-step instructions on how to apply for SBIR and STTR grants.