Congressional Justification FY 2021
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health (NCCIH)
On this page:
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Mechanism Table
- Major Changes in Budget Request
- Summary of Changes
- Budget Graphs
- Budget Authority by Activity
- Authorizing Legislation
- Appropriations History
- Justification of Budget Request
- Budget Authority by Object Class
- Salaries and Expenses
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
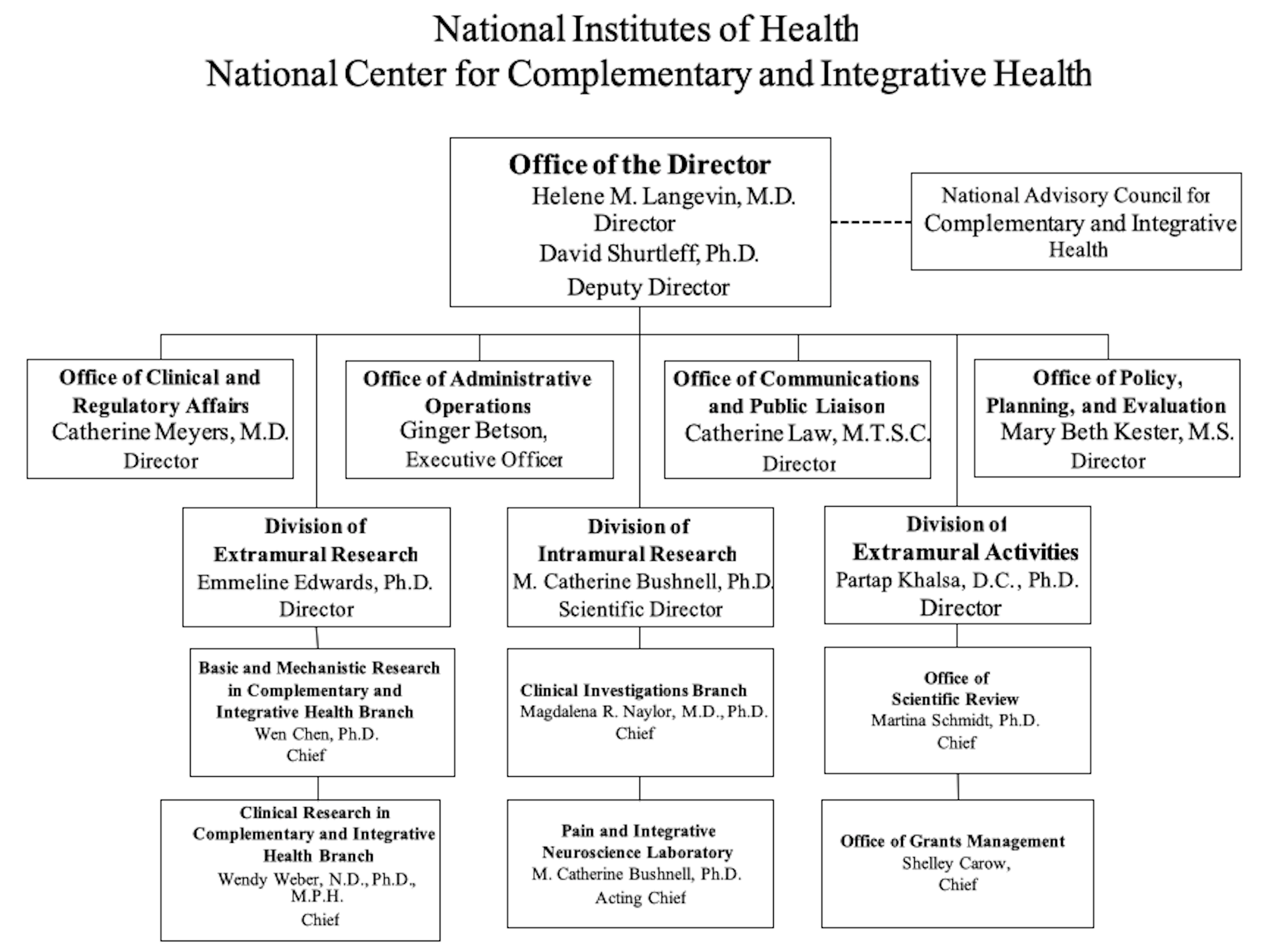
NATIONAL INSTITUTES OF HEALTH
NATIONAL CENTER FOR COMPLEMENTARY AND INTEGRATIVE HEALTH
For carrying out section 301 and title IV of the PHS Act with respect to complementary and integrative health, [$151,740,000]$138,167,000.
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | FY 2019 Final | FY 2020 Enacted | FY 2021 President’s Budget |
|---|---|---|---|
| Appropriation | $146,473 | $151,740 | $138,167 |
| Mandatory Appropriation: (non-add) | |||
| Type 1 Diabetes | (0) | (0) | (0) |
| Other Mandatory financing | (0) | (0) | (0) |
| Rescission | 0 | 0 | 0 |
| Sequestration | 0 | 0 | 0 |
| Secretary's Transfer | -503 | 0 | 0 |
| Subtotal, adjusted appropriation | $145,970 | $151,740 | $138,167 |
| OAR HIV/AIDS Transfers | -9 | 137 | 0 |
| HEAL Transfer from NINDS | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $145,961 | $151,877 | $138,167 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $145,961 | $151,877 | $138,167 |
| Unobligated balance lapsing | -28 | 0 | 0 |
| Total obligations | $145,933 | $151,877 | $138,167 |
| 1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account: FY 2019 — $788 FY 2020 — $1,320 FY 2021 — $1,222 | |||
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Budget Mechanism - Total 1
(Dollars in Thousands)
| MECHANISM | FY 2019 Final | FY 2020 Enacted | FY 2021 President’s Budget | FY 2021 +/- FY 2020 Enacted | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Noncompeting | 125 | $62,460 | 140 | $70,070 | 129 | $64,625 | -11 | $5,445 |
| Administrative Supplements | (18) | 2,462 | (0) | 900 | (0) | 500 | (0) | -400 |
| Competing: | ||||||||
| Renewal | 1 | 792 | 0 | 0 | 0 | 0 | 0 | 0 |
| New | 50 | 20,040 | 44 | 17,874 | 42 | 17,033 | -2 | -841 |
| Supplements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Competing | 51 | $20,832 | 44 | $17,874 | 42 | $17,033 | -2 | -$841 |
| Subtotal, RPGs | 176 | $85,754 | 184 | $88,845 | 171 | $82,158 | -13 | -$6,687 |
| SBIR/STTR | 13 | 4,201 | 13 | 4,335 | 12 | 3,801 | -1 | -534 |
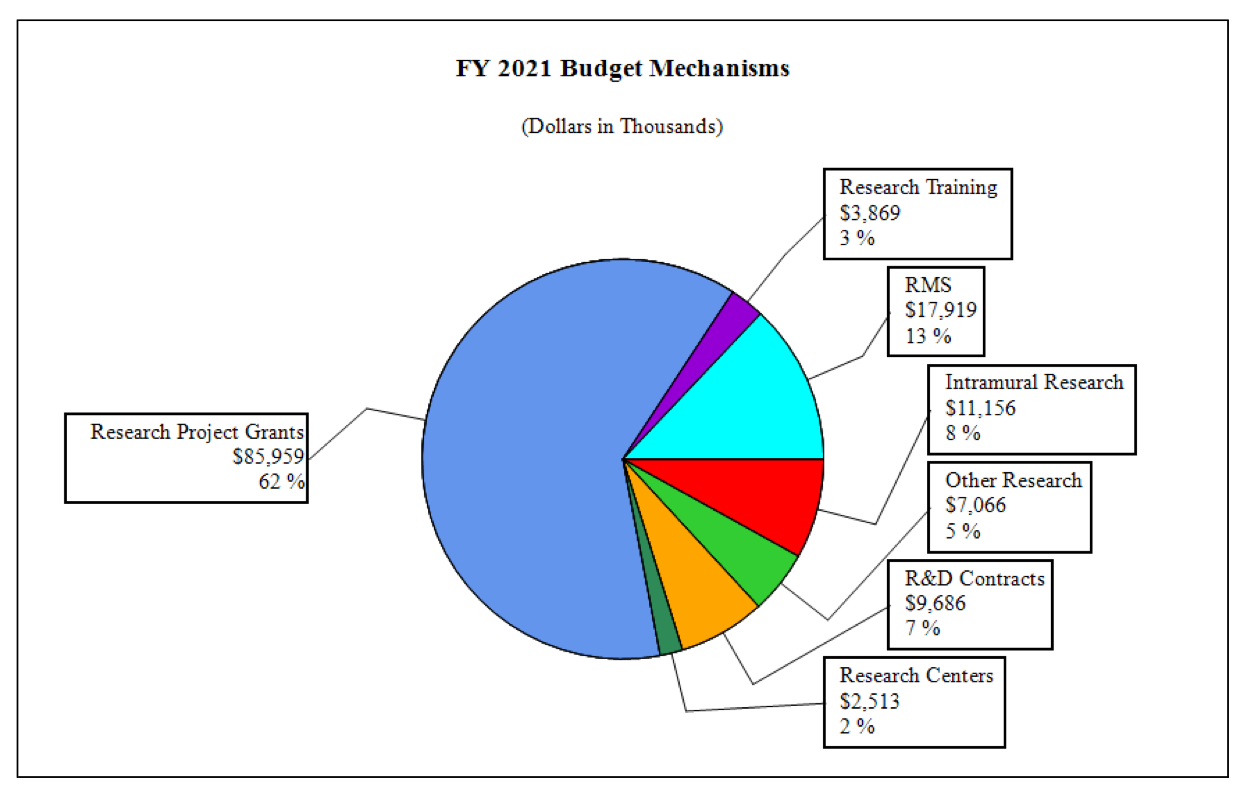
| Research Project Grants | 189 | $89,955 | 197 | $93,180 | 183 | $85,959 | -14 | -$7,221 |
| Research Centers: | ||||||||
| Specialized/Comprehensive | 4 | $4,533 | 4 | $3,362 | 3 | $2,513 | -1 | -$849 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 2 | 840 | 0 | 0 | 0 | 0 | 0 | -0 |
| Comparative Medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 6 | $5,373 | 4 | $3,362 | 3 | $2,513 | -1 | -$849 |
| Other Research: | ||||||||
| Research Careers | 35 | $5,850 | 34 | $5,684 | 22 | $3,719 | -12 | -$1,965 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 9 | 2,227 | 11 | 2,731 | 14 | 3,347 | 3 | 615 |
| Other Research | 44 | $8,077 | 45 | $8,415 | 36 | $7,066 | -9 | -$1,350 |
| Total Research Grants | 239 | $103,404 | 246 | $104,957 | 222 | $95,537 | -24 | -$9,420 |
| Ruth L Kirchstein Training Awards: | FTTPs | FTTPs | FTTPs | FTTPs | ||||
| Individual Awards | 23 | $1,065 | 25 | $1,158 | 15 | $696 | -10 | -$463 |
| Institutional Awards | 36 | 2,404 | 56 | 3,721 | 48 | 3,173 | -8 | -548 |
| Total Research Training | 59 | $3,469 | 81 | $4,879 | 63 | $3,869 | -18 | -$1,010 |
| Research & Develop. Contracts | 8 | $8,741 | 10 | $10,644 | 9 | $9,686 | -1 | -$958 |
| (SBIR/STTR) (non-add) | (0) | (45) | (0) | (46) | (0) | (42) | (0) | (-4) |
| Intramural Research | 8 | 11,606 | 8 | 12,535 | 8 | 11,156 | 0 | -1,379 |
| Res. Management & Support | 63 | 18,740 | 65 | 18,863 | 65 | 17,919 | 0 | -943 |
| Res. Management & Support (SBIR Admin) (non-add) | (0) | (0) | (0) | (0) | (0) | (0) | (0) | (0) |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
| Total, NCCIH | 71 | $145,961 | 73 | $151,877 | 73 | $138,167 | 0 | -$13,710 |
1 All items in italics and brackets are non-add entries.
Major Changes in the Fiscal Year 2021 President’s Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanisms and activity detail and these highlights will not sum to the total change for the FY 2021 budget request for the NCCIH, which is $138.2 million, a decrease of $13.7 million from the FY 2020 Enacted level. The FY 2021 President’s Budget reflects the Administration’s fiscal policy goals for the Federal Government. Within that framework, NCCIH will pursue its highest research priorities through strategic investments and careful stewardship of appropriated funds.
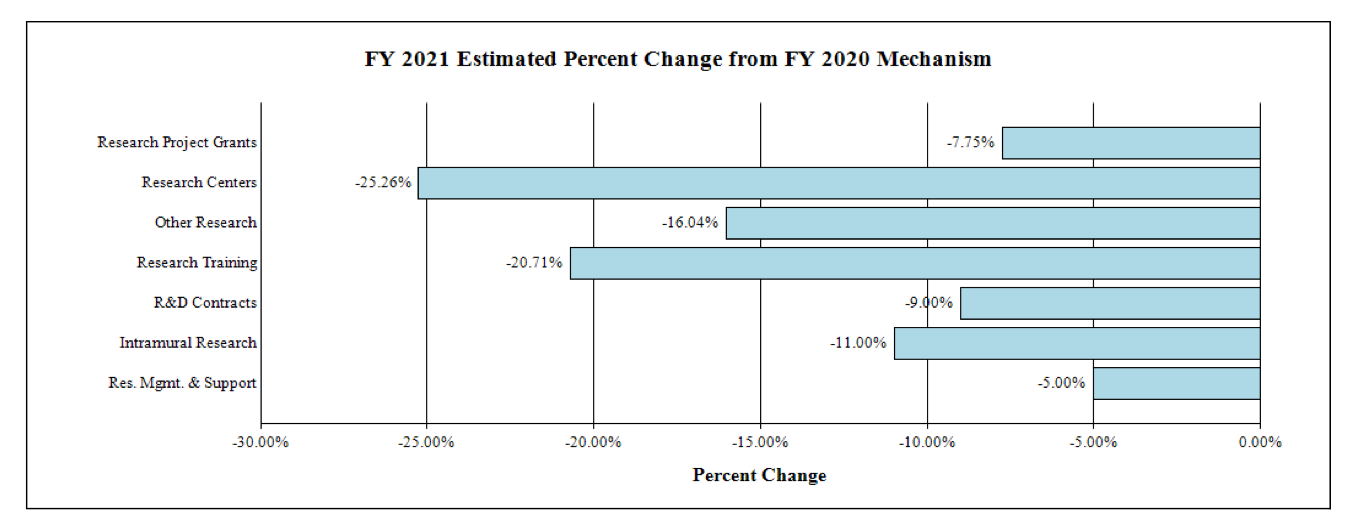
Research Project Grants (-$7.2 million; total $86.0 million): NCCIH will support a total of 183 Research Project Grant (RPG) awards in FY 2021. Noncompeting RPGs will decrease by $5.4 million, while Competing RPG awards will decrease by $0.8 million.
R&D Contracts (-$1.0 million; total $9.7 million): Although the total amount awarded is estimated to decrease in FY 2021, NCCIH will continue its annual support to the National Health Interview Survey (NHIS) and other mission-supporting Research and Development Contracts.
Intramural Research (-$1.4 million; total $11.1 million): NCCIH will continue to support intramural research activities, including Dr. Langevin’s Research Lab.
Research Management and Support (-$0.9 million; total $17.9 million): NCCIH will maintain a flat full-time equivalent staff level and pay inflation-related costs.
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Summary of Changes
(Dollars in Thousands)
| FY 2020 Enacted | $151,877 | ||||
|---|---|---|---|---|---|
| FY 2021 President’s Budget | $138,167 | ||||
| Net Change | -$13,710 | ||||
| CHANGES | FY 2021 President’s Budget | Changes from FY 2020 Enacted | |||
| FTEs | Budget Authority | FTEs | Budget Authority | ||
| A. Built-in: | |||||
| 1. Intramural Research: | |||||
| a. Annualization of January 2020 pay increase & benefits | $3,335 | $23 | |||
| b. January FY 2021 pay increase & benefits | 3,335 | 45 | |||
| c. Paid days adjustment | 3,335 | -12 | |||
| d. Differences attributable to change in FTE | 3,335 | 0 | |||
| e. Payment for centrally furnished services | 1,867 | -366 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 5,954 | 74 | |||
| Subtotal | -$237 | ||||
| 2. Research Management and Support: | |||||
| a. Annualization of January 2020 pay increase & benefits | $11,372 | $72 | |||
| b. January FY 2021 pay increase & benefits | 11,372 | 181 | |||
| c. Paid days adjustment | 11,372 | -42 | |||
| d. Differences attributable to change in FTE | 11,372 | 0 | |||
| e. Payment for centrally furnished services | 577 | -88 | |||
| f. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | 5,971 | 83 | |||
| Subtotal | $205 | ||||
| Subtotal, Built-in | -$31 | ||||
| CHANGES | FY 2021 President’s Budget | Changes from FY 2020 Enacted | ||
|---|---|---|---|---|
| No. | Amount | No. | Amount | |
| B. Program: | ||||
| 1. Research Project Grants: | ||||
| a. Noncompeting | 129 | $65,125 | -11 | -$5,845 |
| b. Competing | 42 | 17,033 | -2 | -841 |
| c. SBIR/STTR | 12 | 3,801 | -1 | -534 |
| Subtotal, RPGs | 183 | $85,959 | -14 | -$7,221 |
| 2. Research Centers | 3 | $2,513 | -1 | -$849 |
| 3. Other Research | 36 | 7,066 | -9 | -1,350 |
| 4. Research Training | 63 | 3,869 | -18 | -1,010 |
| 5. Research and development contracts | 9 | 9,686 | -1 | -958 |
| Subtotal, Extramural | $109,092 | -$11,388 | ||
| FTEs | FTEs | |||
| 6. Intramural Research | 8 | $11,156 | 0 | -$1,142 |
| 7. Research Management and Support | 65 | 17,919 | 0 | -1,148 |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, Program | 73 | $138,167 | 0 | -$13,679 |
| Total changes | -$13,710 | |||
Fiscal Year 2021 Budget Graphs
History of Budget Authority and FTEs:
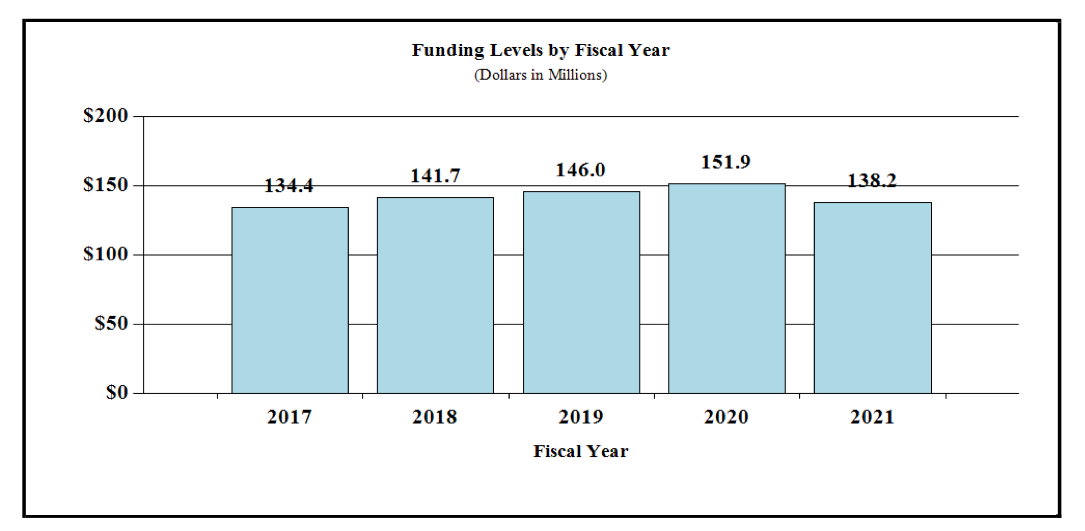
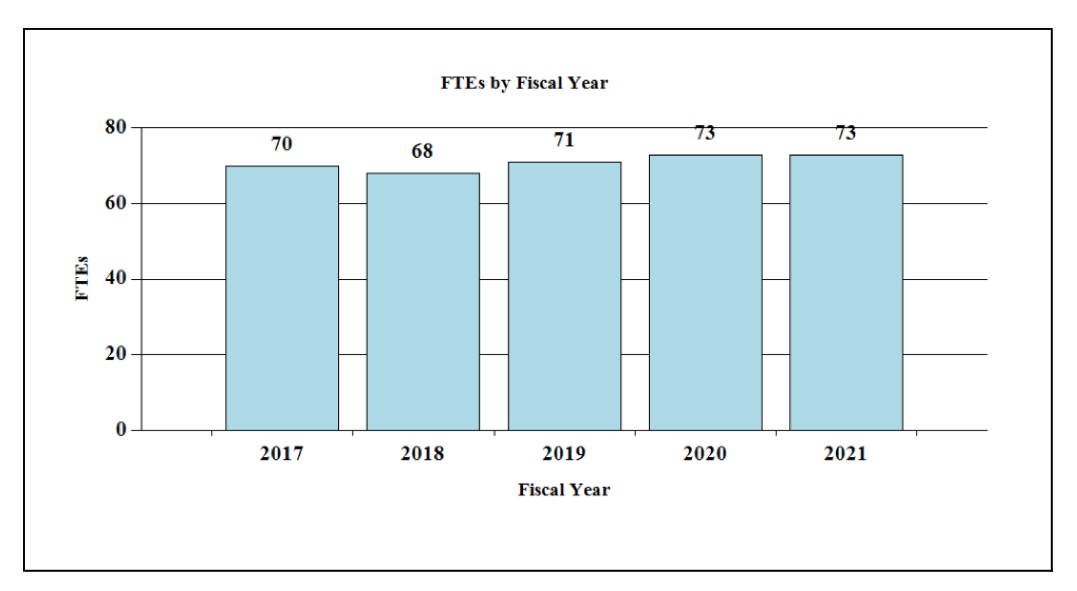
Distribution by Mechanism:
Change by Selected Mechanisms:
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Budget Authority by Activity1
(Dollars in Thousands)
| FY 2019 Final | FY 2020 Enacted | FY 2021 President’s Budget | FY 2021 +/- FY2020 | |||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research | FTE | Amount | FTE | Amount | FTE | Amount | FTE | Amount |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | ||||||||
| Detail | ||||||||
| Clinical Research | $92,108 | $64,093 | $58,165 | -$5,928 | ||||
| Basic Research | 19,337 | 51,508 | 47,058 | -4,450 | ||||
| Extramural Research Training and Capacity Building | 4,170 | 4,879 | 3,869 | -1010 | ||||
| Subtotal, Extramural | $115,614 | $120,480 | $109,092 | -$11,388 | ||||
| Intramural Research | 8 | $11,606 | 8 | $12,535 | 8 | $11,156 | 0 | -$1,379 |
| Research Management & Support | 63 | $18,740 | 65 | $18,863 | 65 | $17,919 | 0 | -$943 |
| TOTAL | 71 | $145,961 | 73 | $151,877 | 73 | $138,167 | 0 | -$13,710 |
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Authorizing Legislation
| PHS Act/Other Citation | U.S. Code Citation | 2020 Amount Authorized | FY 2020 Enacted | 2021 Amount Authorized | FY 2021 President’s Budget | |||
|---|---|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | Indefinite | ||||
| $151,877,000 | $138,167,000 | |||||||
| National Center for Complementary and Integrative Health | Section 401(a) | 42§281 | Indefinite | Indefinite | ||||
| Total, Budget Authority | $151,877,000 | $138,167,000 | ||||||
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation | ||||
|---|---|---|---|---|---|---|---|---|
1 Budget Estimate to Congress includes mandatory financing. | ||||||||
| 2012 | $131,002,000 | $131,002,000 | $126,275,000 | $128,299,000 | ||||
| Rescission | $242,485 | |||||||
| 2013 | $127,930,000 | $128,318,000 | $128,056,515 | |||||
| Rescission | $256,113 | |||||||
| Sequestration | ($6,427,556) | |||||||
| 2014 | $129,041,000 | $128,183,000 | $124,296,000 | |||||
| Rescission | $0 | |||||||
| 2015 | $124,509,000 | $124,681,000 | ||||||
| Rescission | $0 | |||||||
| 2016 | $127,521,000 | $127,585,000 | $130,162,000 | $130,789,000 | ||||
| Rescission | $0 | |||||||
| 20171 | $129,941,000 | $134,549,000 | $136,195,000 | $134,689,000 | ||||
| Rescission | $0 | |||||||
| 2018 | $101,793,000 | $136,741,000 | $139,654,000 | $142,184,000 | ||||
| Rescission | $0 | |||||||
| 2019 | $130,717,000 | $143,882,000 | $146,550,000 | $146,473,000 | ||||
| Rescission | $0 | |||||||
| 2020 | $126,081,000 | $153,632,000 | $154,695,000 | $151,740,000 | ||||
| Rescission | $0 | |||||||
| 2021 | $138,167,000 | |||||||
Justification of Budget Request
National Center for Complementary and Integrative Health
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2019 Enacted | FY 2020 Enacted | FY 2021 President’s Budget | FY 2021 +/- FY 2020 | |
|---|---|---|---|---|
| BA | $145,961,000 | $151,877,000 | $138,167,000 | -$13,710,000 |
| FTE | 71 | 73 | 73 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
The mission of the National Center for Complementary and Integrative Health (NCCIH) is to define, through rigorous scientific investigation, the safety and effectiveness of complementary and integrative health approaches, which include dietary, psychological, and physical approaches that may have originated outside of conventional medicine. This diverse group of health practices includes natural products, such as dietary supplements, plant-based products, and probiotics, as well as mind and body approaches, such as yoga, massage therapy, meditation, mindfulness-based stress reduction, spinal manipulation, and acupuncture. These approaches are considered complementary because they are used in conjunction with conventional medicine. Integrative health care seeks to bring conventional and complementary approaches together in a safe, coordinated way with the goal of improving clinical care for patients, health promotion, and disease prevention.
NCCIH, formerly known as the National Center for Complementary and Alternative Medicine (NCCAM), was created 20 years ago to facilitate the study and evaluation of complementary and alternative medical practices and to disseminate the resulting information to the public. At that time, the use of these practices was growing in popularity and availability, but little was known about their safety and efficacy. In addition, patients rarely discussed their use of complementary approaches with their doctors, and some used these approaches as an alternative to conventional medical care. This created potential problems for patients as some complementary approaches such as natural products can interfere with prescribed medications. In addition, some individuals may use approaches that are safe and effective for some conditions, but not for others. For example, evidence suggests that acupuncture may be effective for back and neck pain, but not for hip osteoarthritis. NCCIH was created to address this scientific and public health need. In the last twenty years, NCCIH has worked to advance the position that these complementary therapies should be “integrated” with and not used as an “alternative” to conventional medicine. The name of the center was changed in 2014 from NCCAM to NCCIH to reinforce this position. The Center has helped build the scientific infrastructure needed to conduct rigorous scientific research of complementary health approaches. The Center has expanded the scientific knowledge base around these practices and established resources to disseminate this information to the public—ultimately impacting their use.
Establishing the scientific infrastructure necessary to study complementary and integrative health approaches: The scientific enterprise requires specially trained scientists, research facilities, and solid evidence. NCCIH has invested in the establishment and maintenance of a scientific infrastructure necessary to support rigorous scientific research on complementary and integrative health approaches. The Center has funded research training and career development grants to increase the number and quality of scientists in this field. NCCIH invested in the development of new tools and methods for evaluating complementary health approaches and their integration into health care. For example, NCCIH is leading the NIH Healthcare Systems Research Collaboratory.1This NIH Common Fund project supports research into pragmatic “real-world” clinical trials. This emerging type of clinical trial involves a real-world distribution of patients in a real-world healthcare setting; however, more knowledge is needed on how to conduct and interpret this type of research. The NCCIH Clinical Research Toolbox2 provides a web-based information repository to facilitate the conduct of high-quality clinical research studies. In addition, the knowledge gained from the NIH Healthcare Systems Research Collaboratory allowed for the formation of the NIH-VA-DoD Pain Management Collaboratory (see program portrait). This partnership is working to investigate nonpharmacologic pain management approaches in the military and veteran populations.
NCCIH has supported the creation of research centers to stimulate collaborations, coordinate resources, and cultivate research and investigators. For example, the Centers for Advancing Research on Botanicals and Other Natural Products (CARBON) Program, launched in 1999 in collaboration with the NIH Office of Dietary Supplements, promotes collaborative and transdisciplinary research on the safety, effectiveness, and mechanisms of action of botanical dietary supplements that have a high potential to benefit human health. The CARBON Program is comprised of three Botanical Dietary Supplements Research Centers, and two Centers for Advancing Natural Products Innovation and Technology. NCCIH has also established new standards for rigor and reproducibility within the field. For example, NCCIH established a natural product integrity policy and quality control program for the study of herbal medicines, dietary supplements, and probiotics. This policy requires all proposed research projects to submit information on the source, composition, and process of production of the product it plans to study. This information is reviewed by experts to ensure that product quality is adequate to yield definitive and reproducible research results. Product information has been evaluated for more than 500 research projects since the policy was established in 2006.
Altogether, NCCIH has funded approximately 1,970 scientific research and training grants, which have contributed to more than 11,000 peer-reviewed scientific publications. These efforts have helped establish a better understanding of what and under which conditions complementary health approaches are safe and effective. NCCIH will continue to invest in building, maintaining, and expanding the scientific infrastructure necessary to conduct this research.
Providing resources to inform decision making: The landscape of complementary and integrative health is inundated with misinformation, some of it overtly promotional, and much of it either not based on scientific evidence or based on information of questionable quality and reliability; therefore, it is important for NCCIH to serve as a source of unbiased, reliable, evidence-based information for the public. The Center provides resources targeted to different types of audiences. For the general consumer, NCCIH has a “Health Topics A-Z”3 series that provides research-based information about complementary health approaches and specific health conditions. The “Herbs at a Glance”4series contains brief fact sheets providing basic information about specific herbs or botanicals—common names, what the science says, potential side effects and cautions, and resources for more information. This information is also available through a user-friendly mobile app calledHerbList™. NCCIH recently launched a new initiative called “Know the Science”5 that seeks to explain scientific topics related to health research. The materials are designed to provide content and engagement for consumers to familiarize them with the science not only on topics in complementary and integrative health, but those common to all areas of health research. For the scientific and medical communities, the Center publishes a monthly e-newsletter called the “NCCIH Clinical Digest”6 that summarizes the state of the science on complementary and integrative health practices for a specific health condition (diabetes, cancer, sleep disorders, etc.). It includes links to relevant clinical guidelines, scientific research, continuing medical education resources, and provides resources for interested patients. All information is freely available on the NCCIH website (nccih.nih.gov) or through the NCCIH Information Clearinghouse. Providing these resources is an important part of the mission of NCCIH and the Center will continue to invest in these efforts.
Impacting use of complementary health approaches: NCCIH was established, in part, to enable the American public to make informed decisions about how, when and for what conditions a complementary health approach should or should not be used. This is important not only from a safety perspective, but also from an economical one. Americans spend approximately $30.2 billion per year on complementary health approaches,7and the results of NCCIH supported studies have influenced their use. Here are a few examples.
Echinacea has traditionally been used for colds, flu and other infections without conclusive evidence to support its use. The National Health Interview Study (NHIS) conducted in 2002 found that 7.6 percent of adults were using echinacea.8 Between 2000 and 2012, NCCIH funded 10 scientific research projects related to echinacea, which contributed to approximately 36 peer-reviewed scientific articles. These studies found that echinacea was relatively safe for use but was not an effective treatment for colds. NCCIH disseminated this information to the public and by 2012 the use of echinacea dropped from 7.6 to 0.9 percent among adults in the United States.9
Fish oils containing omega-3 fatty acid have been reported to reduce the risk of heart disease but there was not conclusive evidence to support their use. The NHIS study found that 2.2 percent of U.S. adults were taking fish oil or omega-3 fatty acid supplements in 2002.8Between 1999 and 2012, NCCIH supported 26 scientific research projects related to fish oil/omega-3 fatty acid supplements, which contributed to more than 200 peer-reviewed scientific publications. These and other studies found that omega-3 supplements do not reduce the risk of heart disease; however, eating seafood one to four times a week can reduce the risk of death due to heart disease. The studies also found that high doses of omega-3 fatty acids can reduce levels of triglycerides and relieve symptoms of rheumatoid arthritis. By 2012 the use of fish oil/omega-3 fatty acids increased from 2.2 to 7.8 percent among adults in the United States and is currently the most commonly used non-vitamin dietary supplement.8
Acupuncture is a key component of traditional Chinese medicine and has been practiced for thousands of years to treat pain and other conditions. Between 1999 and 2018, NCCIH supported 175 scientific research projects related to acupuncture, which have contributed to more than 1,400 peer-reviewed scientific publications. Results from these and other studies suggest that acupuncture can help ease types of chronic pain such as low-back pain, neck pain, migraine, and osteoarthritis/knee pain. The potential benefits of acupuncture for pain management have been embraced within the military population, which experiences a higher prevalence of chronic pain than the general population. The Military Healthcare Service provides acupuncture services in the clinic and has adapted the technique for easier administration in the field. The Centers for Medicare and Medicaid Services (CMS) have partnered with NCCIH to test acupuncture for the treatment of chronic low back pain in patients 65 years of age or older in a pragmatic “real-world” clinical trial funded as part of NIH’s Helping to End Addiction Long TermSM (HEAL) initiative. Results of the study will provide CMS with evidence needed to inform their coverage determinations for acupuncture in older adults.
NCCIH has contributed to many clinical trials in the last 20 years. Clinical trials are the culmination of decades of scientific research. These trials are costly but can result in new treatment strategies (see program portrait on the impact of long-term scientific research, below).
Advancing nonpharmacologic approaches for pain management: A recent study conducted by NCCIH scientists found that the percentage of U.S. adults suffering from a painful condition has increased significantly from 120.2 million (32.9 percent) in 1997/1998 to 178 million (41 percent) in 2013/2014. Furthermore, the use of strong opioids, like fentanyl, morphine, and oxycodone, for pain management among adults with severe pain that interferes with daily activities has also increased from 4.1 million (11.5 percent) in 2001/2002 to 10.5 million (24.3 percent) in 2013/2014. This analysis was based on 18 years of Medical Expenditure Panel Survey data.10 Pain is an enormous public health problem that costs more than $600 billion per year in treatments and lost productivity.11 Effective pain management is a major medical challenge in the United States as the current drug-based treatments are only partially effective and can have serious side effects. As a result, pain is one of the leading reasons Americans turn to complementary health approaches.7 NCCIH is responding to this public health need and devotes approximately 40 percent of its budget to research related to pain and pain management.
NCCIH works to advance knowledge on the basic biology of pain. The Center’s Intramural Research Program conducts basic, clinical, and translational research focusing on the role of the brain in perceiving, modifying, and managing pain. Recently, a team of NCCIH researchers investigated how specific cell-types within the central nucleus of the amygdala contribute to pain perception. The central amygdala is a region of the brain associated with emotional processes and functions as a pain center. This region is ideally positioned to link experience, context, and emotional states with behavioral responses to painful stimuli in both normal and disease states. In a new study, researchers found a cellular “switch” that can both turn up or turn down pain signals. The “switch” acts like a “pain rheostat,” like a home thermostat that regulates temperature—the pain rheostat reacts to pain signals to regulate pain sensations.12Previous research has shown that emotional states can impact pain severity. These new data present an intriguing possibility that emotion could influence the pain rheostat in the central amygdala. More research is needed to evaluate if emotion impacts this pain rheostat and if so, how.
In another study, NCCIH scientists explored the function of a cellular receptor called Piezo2 and its role in detecting touch and pain. Investigators “knocked out” the Piezo2 gene in a large subset of mouse sensory neurons and found that the nerve cells didn’t respond to gentle touch but responded strongly to pinching. These findings were also observed in humans with a non-functional Piezo2 gene.13,14The ability of Piezo2 to respond to gentle but not painful touch could potentially be utilized in the treatment of tactile allodynia, a condition in which typically non-painful touch causes pain. Blocking Piezo2 activity would still preserve the body’s ability to detect harmful pain.
NCCIH is also leading an effort to establish an Intramural Pain Research Center within the NIH Clinical Center (CC). Participating ICs include the National Insitute of Neurological Disorders and Stroke (NINDS), the National Institute of Nursing Research (NINR), the National Institute of Dental and Craniofacial Research (NIDCR), the National Heart, Lung, and Blood Institute (NHLBI), the National Institute on Aging (NIA), the National Cancer Institute (NCI), the National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), the National Institute on Minority Health and Health Disparities (NIMHD), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the Office of Research on Women’s Health (ORWH) and the CC. This evolving, multidisciplinary initiative will help identify specific pain mechanisms, determine the efficacy of non-opioid treatments, and predict individual patient response to therapies and outcomes. The Pain Research Center concept is still under development, but initial efforts have focused on the urgent need to develop a pain phenotyping and brain imaging platform to support project-specific initiatives and serve the many patients at the Clinical Center who are experiencing intractable pain associated with their disease and/or treatment. The Pain Research Center also plans to collect a wide range of measures related to pain, thus allowing the NIH medical community to quantify patients’ pain and target it with effective novel non-opioid interventions. Three projects are underway as interested NIH Institutes and Centers continue to develop the infrastructure for this collaborative research center. These projects are exploring the mechanisms of persistent chronic pain following recovery from sickle cell disease, the psychosocial and neural factors that shape pain responses and opioid use following third molar extraction surgery, and the impact of genetics and neurobiology on pain processing.
NCCIH is expanding its efforts around natural products for pain management. Natural products have historically been a source of novel pain-relieving compounds developed into pharmaceuticals (e.g., willow bark into aspirin). In fact, much of what we know about pain detection is due to discovery of natural products that illuminated the various signaling pathways and targets responsible for sensory perception of painful stimuli. NCCIH plans to develop a coordinated platform of activities that will increase discovery of novel nonaddictive analgesic natural products. These efforts include increasing the knowledge base of natural products traditionally used for pain management around the world, screening activities to identify key compounds, target validation, preclinical Investigational New Drug application enabling research, and clinical trials on new nonaddictive pain management treatments. The cannabis plant is one natural product with potential pain-relieving properties. Cannabis is a unique source of phytochemicals, containing over 100 cannabinoids and terpenes, each with its own pharmacology. The cannabinoid THC is the most widely studied and is responsible for the plant’s addictive and psychoactive effects; however very few of the other cannabinoids and terpenes have been extensively studied. NCCIH recently funded nine new research awards that will investigate the potential pain-relieving properties and mechanisms of actions of the diverse phytochemicals in cannabis, including both minor cannabinoids (non-THC) and terpenes. This investment of approximately $15 million over 5 years will strengthen the evidence regarding cannabis components and whether they have potential roles in pain management.
NCCIH supports research on behavioral strategies to manage chronic pain and improve adherence to the medical treatment of opioid use disorders and reduce the psychological and physical cravings to use opioids. This is especially important in patients who have successfully quit using opioids, but who continue to have pain and need strategies for pain management that will not increase their likelihood of relapse. Medication assisted treatment continues to be the most effective treatment for opioid use disorder. Unfortunately, nearly half of individuals in ongoing methadone maintenance treatment (a common form of medication assisted treatment) use opioids during treatment or relapse within several months.15Chronic pain contributes to the continued use of opioids and relapse. A new study supported by NCCIH evaluated the effects of mindfulness-oriented recovery enhancement (MORE) on opioid cravings and chronic pain. MORE is an integrative behavioral group therapy that provides instruction on mindfulness techniques to increase awareness of and self-control over cravings and foster nonreactivity to emotional and physical pain; reappraisal skills to promote emotion regulation and restructure motivations for opioid use; and savoring pleasant events and emotions to remediate deficits in natural reward processing and boost positive affectivity. The eight-week preliminary study found that participants in the MORE group reported significantly greater decreases in opioid cravings, pain unpleasantness, and stress than those who received treatment as usual. Participants in the MORE group also reported having greater self-control over cravings (129 percent) than participants in the treatment-as-usual group.16 These results are promising but were based on a small number of participants. The researchers are expanding their study to include more participants and an upcoming study of MORE will assess days of opioid use and time in methadone maintenance therapy as outcomes. To advance this type of research, NCCIH is leading the Helping to End Addiction Long-Term (HEAL) initiative’s Behavioral Research to Improve Medication Assisted Treatment program, which seeks to understand whether adding behavioral interventions such as cognitive behavioral therapy or meditation approaches can help improve outcomes for patients with opioid use disorder (OUD) by helping them stay on their medications for treating OUD. NCCIH is overseeing 14 HEAL grants in this area of research.
NCCIH is investing in pragmatic clinical trials of nonpharmacologic approaches for pain management. Pragmatic clinical trials are human effectiveness trials that can be embedded into standard healthcare. These studies are beneficial because they are conducted in a real-world setting with a real-world distribution of patients. NCCIH is leading the NIH-Department of Defense-Veterans Affairs Pain Management Collaboratory (PMC). This partnership between NCCIH and other Federal agencies supports research evaluating the efficacy of nonpharmacologic pain management approaches within the military and veteran healthcare systems and investigating how these approaches could be integrated into the healthcare systems (see program portrait). NCCIH is also leading the HEAL initiative’s Pragmatic and Implementation Studies for the Management of Pain to Reduce Opioid Prescribing program, which seeks to take interventions and treatment guidelines that have already been shown to work for specific pain conditions and integrate them into healthcare delivery systems. The Center is overseeing three HEAL grants in this area of research.
Promoting the health of the whole person: The second most common reason Americans use complementary health approaches is to promote health and wellness. While a lot of natural products sold as dietary supplements claim to promote heath or prevent disease, there is little to no evidence to support their use. However, one study supported by NCCIH found potential neuroprotective effects of coffee in Parkinson’s disease. Previous research had linked coffee consumption to a reduced risk of Parkinson’s disease, and caffeine is generally believed to be the protective agent. However, coffee is a complex chemical mixture containing more than a thousand different substances and several lines of evidence suggest that other components within coffee may also play a neuroprotective role. In this new study, two coffee components, eicosanoyl-5-hydroxytryptamide (EHT) and caffeine, were evaluated separately and together in mouse models of Parkinson’s disease. The researchers found that individually these compounds did not have an effect; however, when given together mice exhibited better nerve cell function, less nerve inflammation, and closer-to-normal behavior.17 These data suggest that the composition of coffee is important for its neuroprotective effects in animal models of Parkinson’s disease. As more is learned, it may become possible to optimize the composition of coffee to enhance its effects.
Another area of interest is the role of mind and body approaches in health. Individual behavior plays a key role in health promotion and disease prevention. We know that a combination of poor diet, sedentary lifestyle, and chronic stress leads to major chronic conditions including cardiovascular disease, diabetes, degenerative joint disease, chronic pain and depression, often occurring in the same person. Behavioral methods such as simple relaxation techniques, especially when taught early in life, can equip patients with tools to help themselves with stress, pain, and sleep for the rest of their lives. More research is needed to understand how certain complementary health approaches can be useful in encouraging better self-care, improving a personal sense of well-being, promoting a greater commitment to a healthy lifestyle, and preventing the onset of mental health conditions (e.g., anxiety and depression).
It is also important to understand health as a process involving the whole person and how complementary health approaches may influence it. Our current biomedical research model is superb in advancing the specialized treatment of organ-specific diseases with increasing precision; however, health and disease impact and rely on multiple systems within the body. Our current understanding of how these various systems influence each other in healthy and diseased conditions is lacking. There is a need to understand health as a process involving the whole person. In addition, complementary health approaches such as yoga, mindfulness mediation, and Tai Chi impact multiple systems of the body (e.g., respiratory, neural, and muscular).
Overall Budget Policy:
The FY 2021 President’s Budget request is $138.2 million, a decrease of $13.7 million or 9.0 percent compared with the FY 2020 Enacted level.
1https://rethinkingclinicaltrials.org/
2https://nccih.nih.gov/grants/toolbox
3https://nccih.nih.gov/health/atoz.htm
4https://nccih.nih.gov/health/herbsataglance.htm
5https://nccih.nih.gov/health/know-science
6https://nccih.nih.gov/health/providers/digest
7 Expenditures on complementary health approaches: United States, 2012. National Health Statistics Reports; no. 95, 2016. OMID: 2735222.
8 Complementary and Alternative Medicine Use Among Adults: United States, 2002. Advance data from vital and health statistics; no. 343, 2004.
9 Trends in the use of complementary health approaches among adults: United States, 2002-2012. National health statistics reports; no 79, 2015
10Eighteen-year trends in the prevalence of, and health care use for, noncancer pain in the United States; Data from the Medical Expenditure Panel Survey. J Pain. 2019.
11Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Institute of Medicine, 2011.
12Dual and opposing function of the central amygdala in the modulation of pain. Cell Reports. 2019.
13The molecular basis of injury-related tactile pain.Sci Transl Med. 2018.
14The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med. 2018.
15A Prospective Study to Investigate Predictors of Relapse among Patients with Opioid Use Disorder Treated with Methadone.Subst Abuse. 2016.
16Mindfulness-oriented recovery enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: ecological momentary assessments from a stage 1 randomized controlled trial.Drug and Alcohol Dependence. 2019.
17Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson’s disease and DLB. PNAS, 2018.
Program Descriptions and Accomplishments
Extramural Clinical Research: The NCCIH extramural research program funds clinical investigations on complementary and integrative health practices and interventions. Projects range from small pilot studies to large-scale clinical trials and epidemiologic studies, including several collaborations between NIH ICs and other Federal agencies.
Budget Policy:
The FY 2021 President’s Budget request is $58.2 million, a decrease of $5.9 million or 9.0 percent compared with the FY 2020 Enacted level.
Program Portrait – The NIH-VA-DoD Pain Management Collaboratory: An Update
Pain is the most common medical condition requiring treatment for military personnel. Studies report nearly 45 percent of soldiers and 50 percent of veterans experience pain on a regular basis, and there is significant overlap among chronic pain, post-traumatic stress disorder (PTSD), and persistent post-concussive symptoms. Data from the 2010-2014 National Health Interview Survey shows that American veterans experience a higher prevalence of pain and more severe pain than non-veterans.18Although opioids are often prescribed to treat chronic pain, there is no evidence to suggest that they are effective, and they are often associated with severe adverse effects and may lead to drug addiction, overdose, and death. Therefore, there is a need for nondrug approaches to complement current strategies for pain management and to reduce the need for, and hazards of, excessive reliance on opioids.
In 2017, NCCIH partnered with the Department of Defense (DoD), the Department of Veterans Affairs (VA), and seven other Institutes and Centers at the NIH to launch the NIH-DoD-VA Pain Management Collaboratory (PMC). The PMC seeks to support the development, implementation, and testing of cost-effective, large-scale, real-world research on nonpharmacologic approaches for pain management and related conditions in military and veteran health care delivery organizations. The PMC is currently supporting 11 pragmatic, large-scale clinic trials within the military and veteran health care delivery organizations. Of these trials, the NIH is supporting six, the DoD is supporting four and the VA is supporting one. Examples of interventions being investigated for their effectiveness in pain management include: cognitive behavioral therapy delivered by phone, stepped care management, behavioral health consultation in primary care, manual therapy such as chiropractic care, and percutaneous peripheral nerve stimulation. These awards are funded through a phased mechanism. This means that an awardee has a 2-year period to plan and test the feasibility of their study design. After this time, they must meet specific milestones in order to move on to the larger, more comprehensive implementation phase of the award. The award is terminated if an investigator fails to meet their milestones. This phased mechanism helps ensure only studies with the highest likelihood of success will continue. The 2-year planning and feasibility phase of the clinical trials ended in FY 2019 and all 11 have met their milestones. The trials transitioned to the implementation phase in FY 2019 and 2020 and will run through FY 2024. The NIH is also supporting a coordinating center that provides technical, design, and other support to the research teams during this demonstration phase and will disseminate Collaboratory-endorsed policies, best practices, and lessons learned from the demonstration projects.
All the studies supported by the PMC will not only show if specific nonpharmacologic approaches are effective for pain management, but also how they can be integrated into a healthcare system. For example, researchers at Yale University are investigating the effect of early resource education on pain management. The investigators in this study are enrolling veterans when they are seeking disability for a pain condition and educating them on the different types of pain medications. In addition, they inform the veterans of the importance of treating both physical and psychological aspects of pain and connect the veterans with the services available to them. Researchers also assess the risk for substance use disorders and depression and refer the veterans to the appropriate treatment. If this intervention is successful, it can be quickly scaled up and made available nationwide to veterans seeking disability. This early education and referral paradigm could also be adapted to other healthcare systems.
Program Portrait – The impact of long-term scientific research
Scientific research is a long-term endeavor with the goal of improving public health and healthcare. Over the last 20 years, NCCIH has started numerous clinical trials, which were built upon countless previous scientific investigations. This program portrait looks back at a few of these trials and evaluates the impacts.
The AREDS trials: The Age-Related Eye Disease Study (AREDS) began in 1992 before NCCIH was created. This clinical trial was sponsored by the National Eye Institute (NEI) and sought to evaluate the effects of a nutritional supplement, called the AREDS formulation, on the progression of age-related macular degeneration (AMD). AMD is one of the leading causes of visual impairment and blindness in the United States. The results from this study were published in 2001 and showed that the AREDS formulation significantly reduced the risk of advanced AMD and its associated vision loss.19 These results were exciting and represented the first intervention shown to reduce the risk of advanced AMD. The AREDS formulation contains high doses of vitamin C, vitamin E, beta-carotene and zinc, and while it was shown to be effective there were concerns about the high concentration of beta-carotene. Previous studies had shown that high concentrations of beta-carotene were associated with an increased risk of lung cancer in smokers. So, in 2006 a second clinical trial was launched called AREDS2 to determine if beta-carotene could be removed from the AREDS formulation and still be effective. NCCIH contributed approximately $1.5 million to this study. The effort was led by NEI and received additional funds from other NIH Institutes. In the AREDS2 trial, the antioxidants lutein and zeaxanthin, which are in the same family of nutrients as beta-carotene, were added to the AREDS1 formulation as a substitute for beta-carotene. The study found that lutein and zeaxanthin together appeared to be a safe and effective alternative to beta-carotene.20The AREDS trials helped identify nutritional supplements that reduced the risk of developing advanced stages of AMD by about 25 percent and the risk of central vision loss by 19 percent in people with high risk of developing the disease.20,21The AREDS1 and 2 formulations have undergone phase 3 clinical trials and are available over-the-counter in the United States.
The TACT trials: The Trial to Assess Chelation Therapy (TACT) began in 2002. This clinical trial was sponsored by NCCIH and the National Heart, Lung, and Blood Institute (NHLBI). The trial sought to determine the safety and efficacy of EDTA (ethylene diamine tetra-acetic acid) chelation therapy in individuals with coronary artery disease—the leading cause of death for both men and women in the United States. Chelation is a chemical process where a substance is used to bind metals or minerals. The FDA has approved chelation with EDTA for the treatment of lead poisoning or exposure to other heavy metals. Before the TACT clinical trial, some physicians and alternative medicine practitioners were recommending EDTA chelation as a complementary treatment for heart disease, without evidence to support its safety or efficacy and sometimes in lieu of proven conventional therapies. The 5-year TACT study was designed to determine if this practice was safe and effective. The results of this study showed the EDTA chelation therapy resulted in a modest reduction in cardiovascular events overall. However, among participants with diabetes there was an impressive 41 percent reduction in the risk of any cardiovascular event; a 52 percent reduction in recurrent heart attacks; and a 43 percent reduction in death from any cause.21In 2016, NCCIH, NHLBI, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the National Institute of Environmental Health Sciences (NIEHS) supported a large follow-up study. The goal of this study, called TACT2, was to repeat the results of the first TACT study—but only in patients with diabetes and a prior heart attack—to see if the apparent benefit could be confirmed. It is anticipated that the TACT2 phase 3 clinical trial will be complete in FY 2021. In total, NCCIH has invested approximately $54.9 million in the TACT trials. These trials will help the FDA determine whether disodium EDTA chelation therapy should be an approved intervention to reduce the risk of further cardiovascular events in patients who have both coronary artery disease and diabetes.
The Cytisine trial: In 2015, NCCIH started a public-private partnership with Achieve Life Sciences, Inc., to advance clinical trials of Cytisine, a natural product for smoking cessation. Cytisine is isolated from the plant Laburnum anagyroides and has been used as a smoking cessation aid, primarily in eastern European countries, for several decades. Cytisine clinical trials conducted outside the United States showed promise in helping participants stop smoking; however, FDA-approved clinical trials needed to be conducted in the United States. Achieve Life Sciences, Inc., was interested in bringing Cytisine to the United States, but was struggling to find private investment to support the pre-clinical safety and toxicology studies needed to begin U.S. clinical trials. NCCIH decided to help Achieve Life Sciences and supported the necessary pre-clinical trials and utilized the NIH Blueprint Neurotherapeutic Network to conduct the studies. This approximately $1.7 million investment enabled FDA acceptance of the Investigational New Drug application for cytisine and for Achieve Life Sciences, Inc., to begin clinical trials and raise private funds in order to conduct them. Since 2015, Achieve Life Sciences, Inc., has successfully completed a phase 2b clinical trial and began phase 3 at the end of 2019. These trials may lead to the wide availability of a new smoking cessation option to address the major public health issues associated with tobacco use.
- 18Severe pain in veterans: the impact of age and sex, and comparisons to the general population. J Pain, 2017.
- 19A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zine for age-related macular degeneration and vision loss: AREDS report no. 8.Arch Ophthalmol. 2001.
- 20Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013.
- 21The effect of an EDTA-based chelation regimen on patients with diabetes and prior myocardial infarction in Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014.
Extramural Basic Research: Basic research on the fundamental biological effects of active components of interventions is central to the development of the evidence base on complementary and integrative health approaches and underpins the design of clinical research. While NCCIH continues its broad support of investigator-initiated research, the Center will also support translational research to optimize therapeutic effects through targeted initiatives. For example, NCCIH supports translational research to ascertain the biological effects of nonpharmacologic therapies on the brain and to optimize their effects. NCCIH is also energizing the research community to overcome the methodologic and technologic obstacles hindering basic research on natural products and nonpharmacologic therapies, as well as encouraging the incorporation of cutting-edge technologies to monitor and enhance these interventions.
Budget Policy:
The FY 2021 President’s Budget request is $47.1 million, a decrease of $4.5 million or 9.0 percent compared with the FY 2020 Enacted level.
Extramural Research Training and Capacity Building:Improving the capacity of the field to carry out rigorous research on complementary and integrative health approaches is a priority for the NCCIH. To increase the number, quality, and diversity of investigators conducting research on complementary and integrative health approaches, NCCIH supports a variety of training and career development activities for pre- and post-doctoral students, and early career investigators. NCCIH also supports workshops to help students and fellows connect to NIH funding opportunities, understand how to interact with NIH staff to develop research proposals, navigate the NIH peer-review process successfully, develop resilience to overcome career roadblocks, and develop plans for a successful research career.
Budget Policy:
The FY 2021 President’s Budget request is $3.9 million, a decrease of $1.0 million or 21.0 percent compared with the FY 2020 Enacted level.
Intramural Research: NCCIH’s intramural research program is focused on understanding the central mechanisms of pain and its modulation, with the long-term goal of improving clinical management of chronic pain through the integration of pharmacologic and nonpharmacologic approaches. Among topics of interest are the pathways and mechanisms by which expectations, emotions, attention, environment, and genetics modulate pain or pain processing.
Budget Policy:
The FY 2021 President’s Budget request is $11.2 million, a decrease of $1.4 million or 11.0 percent compared with the FY 2020 Enacted level.
Research Management Support (RMS): Through its RMS activities, NCCIH provides administrative, budgetary, logistical, and scientific support in the review, award, monitoring, and management of research grants, training awards, and contracts. The Center is migrating to Qlik Sense, which provides interactive reports and enhanced dashboards to provide NCCIH leadership with trend data and performance indicators to ensure responsible stewardship of public funds. The Center continues to improve onboarding and new employee orientation to ensure smooth transition of incoming staff. As part of its outreach efforts, NCCIH disseminates objective, evidence-based information to the public, scientists, and health care providers to help them make informed decisions about the use of complementary and integrative health practices.
Budget Policy:
The FY 2021 President’s Budget request is $17.9 million, a decrease of $0.9 million or 5.0 percent compared with the FY 2020 Enacted level.
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Budget Authority by Object Class1
(Dollars in Thousands)
| FY 2020 Enacted | FY 2021 President’s Budget | FY 2021 +/- FY 2020 | |
|---|---|---|---|
| Total compensable work years: | |||
| Full-time equivalent | 73 | 73 | 0 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 |
| Average ES salary | $0 | $0 | $0 |
| Average GM/GS grade | 13.7 | 13.8 | 0.1 |
| Average GM/GS salary | $128 | $129 | $1 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) | $113 | $113 | $0 |
| Average salary of ungraded positions | $75 | $75 | $0 |
| OBJECT CLASSES | FY 2020 Enacted | FY 2021 President’s Budget | FY 2021 +/- FY 2020 |
| Personnel Compensation | |||
| 11.1 Full-Time Permanent | 7,042 | 7,123 | 81 |
| 11.3 Other Than Full-Time Permanent | 2,340 | 2,367 | 27 |
| 11.5 Other Personnel Compensation | 290 | 293 | 3 |
| 11.7 Military Personnel | 232 | 238 | 6 |
| 11.8 Special Personnel Services Payment | 971 | 983 | 11 |
| 11.9 Subtotal Personnel Compensation | $10,874 | $11,003 | $129 |
| 12.1 Civilian Personnel Benefits | 3,430 | 3,564 | 134 |
| 12.2 Military Personnel Benefits | 137 | 140 | 4 |
| 13.0 Benefits to Former Personnel | 0 | 0 | 0 |
| Subtotal Pay Costs | $14,441 | $14,707 | $266 |
| 21.0 Travel & Transportation of Persons | 491 | 449 | -42 |
| 22.0 Transportation of Things | 31 | 28 | -3 |
| 23.1 Rental Payments to GSA | 19 | 18 | -2 |
| 23.2 Rental Payments to Others | 0 | 0 | 0 |
| 23.3 Communications, Utilities & Misc. Charges | 53 | 49 | -4 |
| 24.0 Printing & Reproduction | 0 | 0 | 0 |
| 25.1 Consulting Services | 1,018 | 448 | -571 |
| 25.2 Other Services | 3,108 | 2,528 | -580 |
| 25.3 Purchase of goods and services from government accounts | 16,282 | 15,006 | -1,275 |
| 25.4 Operation & Maintenance of Facilities | 110 | 100 | -10 |
| 25.5 R&D Contracts | 5,458 | 4,488 | -971 |
| 25.6 Medical Care | 30 | 28 | -2 |
| 25.7 Operation & Maintenance of Equipment | 402 | 369 | -33 |
| 25.8 Subsistence & Support of Persons | 0 | 0 | 0 |
| 25.0 Subtotal Other Contractual Services | $26,409 | $22,967 | -$3,442 |
| 26.0 Supplies & Materials | 297 | 270 | -27 |
| 31.0 Equipment | 299 | 273 | -26 |
| 32.0 Land and Structures | 0 | 0 | 0 |
| 33.0 Investments & Loans | 0 | 0 | 0 |
| 41.0 Grants, Subsidies & Contributions | 109,836 | 99,406 | -10,430 |
| 42.0 Insurance Claims & Indemnities | 0 | 0 | 0 |
| 43.0 Interest & Dividends | 0 | 0 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal Non-Pay Costs | $137,436 | $123,460 | -$13,976 |
| Total Budget Authority by Object Class | $151,877 | $138,167 | -$13,710 |
| 1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Salaries and Expenses
(Dollars in Thousands)
| OBJECT CLASSES | FY 2020 Enacted | FY 2021 President’s Budget | FY 2021 +/- FY 2020 |
|---|---|---|---|
| Personnel Compensation | |||
| Full-Time Permanent (11.1) | $7,042 | $7,123 | $81 |
| Other Than Full-Time Permanent (11.3) | 2,340 | 2,367 | 27 |
| Other Personnel Compensation (11.5) | 290 | 293 | 3 |
| Military Personnel (11.7) | 232 | 238 | 6 |
| Special Personnel Services Payments (11.8) | 971 | 983 | 11 |
| Subtotal Personnel Compensation (11.9) | $10,874 | $11,003 | $129 |
| Civilian Personnel Benefits (12.1) | $3,430 | $3,564 | $134 |
| Military Personnel Benefits (12.2) | 137 | 140 | 4 |
| Benefits to Former Personnel (13.0) | 0 | 0 | 0 |
| Subtotal Pay Costs | $14,441 | $14,707 | $266 |
| Travel & Transportation of Persons (21.0) | $491 | $449 | -$42 |
| Transportation of Things (22.0) | 31 | 28 | -3 |
| Rental Payments to Others (23.2) | 0 | 0 | 0 |
| Communications, Utilities & Misc. Charges (23.3) | 53 | 49 | -4 |
| Printing & Reproduction (24.0) | 0 | 0 | 0 |
| Other Contractual Services: | |||
| Consultant Services (25.1) | 1,018 | 448 | -571 |
| Other Services (25.2) | 3,108 | 2,528 | -580 |
| Purchases from government accounts (25.3) | 12,521 | 10,645 | -1,877 |
| Operation & Maintenance of Facilities (25.4) | 110 | 100 | -10 |
| Operation & Maintenance of Equipment (25.7) | 402 | 369 | -33 |
| Subsistence & Support of Persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | $17,160 | $14,089 | -$3,071 |
| Supplies & Materials (26.0) | $297 | $270 | -$27 |
| Subtotal Non-Pay Costs | $18,033 | $14,886 | -$3,147 |
| Total Administrative Costs | $32,475 | $29,593 | -$2,881 |
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Detail of Full-Time Equivalent Employment (FTE)
| OFFICE/DIVISION | FY 2019 Final | FY 2020 Enacted | FY 2021 President’s Budget | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Office of Scientific Review | |||||||||
| Direct: | 5 | - | 5 | 5 | - | 5 | 5 | - | 5 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 5 | - | 5 | 5 | - | 5 | 5 | - | 5 |
| Division of Extramural Activities | |||||||||
| Direct: | 11 | - | 11 | 12 | - | 12 | 12 | - | 12 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 11 | - | 11 | 12 | - | 12 | 12 | - | 12 |
| Division of Extramural Research | |||||||||
| Direct: | 8 | - | 8 | 8 | - | 8 | 8 | - | 8 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 8 | - | 8 | 8 | - | 8 | 8 | - | 8 |
| Division of Intramural Research Program | |||||||||
| Direct: | 7 | 1 | 8 | 7 | 1 | 8 | 7 | 1 | 8 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 7 | 1 | 8 | 7 | 1 | 8 | 7 | 1 | 8 |
| Office of Administrative Operations | |||||||||
| Direct: | 13 | 1 | 14 | 14 | 1 | 15 | 14 | 1 | 15 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 13 | 1 | 14 | 14 | 1 | 15 | 14 | 1 | 15 |
| Office of Clinical and Regulatory Affairs | |||||||||
| Direct: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Office of Communications and Public Liaison | |||||||||
| Direct: | 8 | - | 8 | 8 | - | 8 | 8 | - | 8 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 8 | - | 8 | 8 | - | 8 | 8 | - | 8 |
| Office of Grants Management | |||||||||
| Direct: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Office of Policy, Planning, and Evaluation | |||||||||
| Direct: | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 3 | - | 3 | 3 | - | 3 | 3 | - | 3 |
| Office of the Director | |||||||||
| Direct: | 6 | - | 6 | 6 | - | 6 | 6 | - | 6 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 6 | - | 6 | 6 | - | 6 | 6 | - | 6 |
| Total | 69 | 2 | 71 | 71 | 2 | 73 | 71 | 2 | 73 |
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FISCAL YEAR | Average GS Grade | ||||||||
| 2017 | 12.9 | ||||||||
| 2018 | 12.8 | ||||||||
| 2019 | 13.2 | ||||||||
| 2020 | 13.7 | ||||||||
| 2021 | 13.8 | ||||||||
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Detail of Positions1
| GRADE | FY 2019 Final | FY 2020 Enacted | FY 2021 President’s Budget |
|---|---|---|---|
| Total, ES Positions | 0 | 0 | 0 |
| Total, ES Salary | 0 | 0 | 0 |
| GM/GS-15 | 13 | 14 | 14 |
| GM/GS-14 | 12 | 12 | 12 |
| GM/GS-13 | 22 | 22 | 22 |
| GS-12 | 9 | 10 | 10 |
| GS-11 | 2 | 2 | 2 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 0 | 0 | 0 |
| GS-8 | 1 | 1 | 1 |
| GS-7 | 1 | 1 | 1 |
| GS-6 | 0 | 0 | 0 |
| GS-5 | 0 | 0 | 0 |
| GS-4 | 0 | 0 | 0 |
| GS-3 | 0 | 0 | 0 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 60 | 62 | 62 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207) | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 1 | 1 | 1 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 1 | 1 | 1 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 2 | 2 | 2 |
| Ungraded | 25 | 25 | 25 |
| Total permanent positions | 62 | 64 | 64 |
| Total positions, end of year | 87 | 89 | 89 |
| Total full-time equivalent (FTE) employment, end of year | 71 | 73 | 73 |
| Average ES salary | 0 | 0 | 0 |
| Average GM/GS grade | 13.2 | 13.7 | 13.8 |
| Average GM/GS salary | 123,741 | 128,196 | 129,477 |
| 1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||