Congressional Justification FY 2018
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health (NCCIH)
On this page:
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Graphs
- Authorizing Legislation
- Appropriations History
- Justification of Budget Request
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
For carrying out section 301 and title IV of the PHS Act with respect to complementary and integrative health, $101,793,000.
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | FY 2016 Final | FY 2017 Annualized CR | FY 2018 President’s Budget |
|---|---|---|---|
| 1 Excludes the following amounts for reimbursable activities carried out by this account: FY 2016 — $487 FY 2017 — $560 FY 2018 — $600 | |||
| Appropriation | $130,789 | $130,789 | $101,793 |
| Mandatory Appropriation: (non-add) | |||
| Type 1 Diabetes | (0) | (0) | (0) |
| Other Mandatory financing | (0) | (0) | (0) |
| Rescission | 0 | -249 | 0 |
| Sequestration | 0 | 0 | 0 |
| Zika Intra-NIH Transfer | -181 | 0 | 0 |
| Subtotal, adjusted appropriation | $130,608 | $130,540 | $101,793 |
| OAR HIV/AIDS Transfers | -848 | 0 | 0 |
| Subtotal, adjusted budget authority | $129,760 | $130,540 | $101,793 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $129,760 | $130,540 | $101,793 |
| Unobligated balance lapsing | 0 | 0 | 0 |
| Total obligations | $129,760 | $130,540 | $101,793 |
Fiscal Year 2018 Budget Graphs
History of Budget Authority and FTEs:
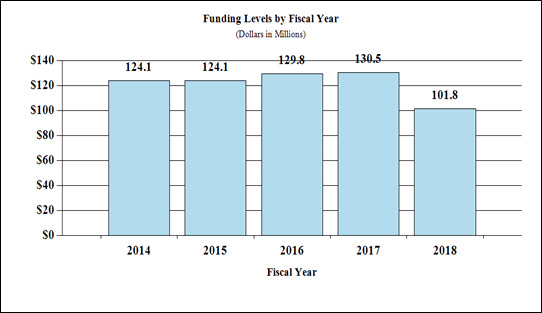
Funding Levels by Fiscal Year
| Fiscal Year | Dollars in Millions |
|---|---|
| 2014 | $124.1 |
| 2015 | $124.1 |
| 2016 | $129.8 |
| 2017 | $130.5 |
| 2018 | $101.8 |
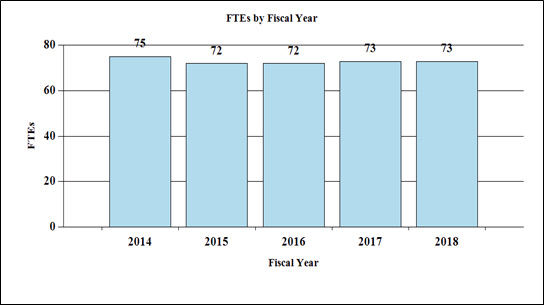
Full-Time Employees by Fiscal Year
| FY | FTEs |
|---|---|
| 2014 | 75 |
| 2015 | 72 |
| 2016 | 72 |
| 2017 | 73 |
| 2018 | 73 |
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Authorizing Legislation
| PHS Act/Other Citation | U.S. Code Citation | 2017 Amount Authorized | FY 2017 Annualized CR | 2018 Amount Authorized | FY 2018 President’s Budget | |||
|---|---|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | Indefinite | ||||
| $130,540,000 | $101,793,000 | |||||||
| National Center for Complementary and Integrative Health | Section 401(a) | 42§281 | Indefinite | Indefinite | ||||
| Total, Budget Authority | $130,540,000 | $101,793,000 | ||||||
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation | ||||
|---|---|---|---|---|---|---|---|---|
1 Budget Estimate to Congress includes mandatory financing. | ||||||||
| 2008 | $121,699,000 | $123,380,000 | $124,213,000 | $121,577,000 | ||||
| Rescission | $2,162,000 | |||||||
| Supplemental | $647,000 | |||||||
| 2009 | $121,695,000 | $125,878,000 | $125,082,000 | $125,471,000 | ||||
| Rescission | $0 | |||||||
| 2010 | $127,241,000 | $129,953,000 | $127,591,000 | $128,844,000 | ||||
| Rescission | $0 | |||||||
| 2011 | $132,004,000 | $131,796,000 | $128,844,000 | |||||
| Rescission | $1,131,327 | |||||||
| 2012 | $131,002,000 | $131,002,000 | $126,275,000 | $128,299,000 | ||||
| Rescission | $242,485 | |||||||
| 2013 | $127,930,000 | $128,318,000 | $128,056,515 | |||||
| Rescission | $256,113 | |||||||
| Supplemental | ($6,427,556) | |||||||
| 2014 | $129,041,000 | $128,183,000 | $124,296,000 | |||||
| Rescission | $0 | |||||||
| 2015 | $124,509,000 | $124,681,000 | ||||||
| Rescission | $0 | |||||||
| 2016 | $127,521,000 | $127,585,000 | $130,162,000 | $130,789,000 | ||||
| Rescission | $0 | |||||||
| 2017 1 | $129,941,000 | $134,549,000 | $136,195,000 | $130,789,000 | ||||
| Rescission | $249,000 | |||||||
| 2018 | $101,793,000 | |||||||
Justification of Budget Request
National Center for Complementary and Integrative Health
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2016 Actual | FY 2017 Annualized CR | FY 2018 President’s Budget | FY 2018 +/- FY 2017 | |
|---|---|---|---|---|
| BA | $129,759,982 | $130,540,000 | $101,793,000 | -$28,747,000 |
| FTE | 72 | 73 | 73 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
The National Center for Complementary and Integrative Health (NCCIH) is the lead Federal agency for scientific research on the usefulness and safety of complementary and integrative health practices. These practices include mind and body interventions, such as massage, acupuncture, yoga, and meditation, and natural products, such as dietary supplements and probiotics. To address the need for objective safety and efficacy evidence on many of these approaches, NCCIH supports rigorous fundamental scientific investigation to better understand how these practices work, for whom, and the optimal methods of practice and delivery. NCCIH is also committed to exploring the potential of complementary health approaches to foster health promotion and disease prevention across the lifespan. NCCIH strives to invest in research that will drive new discoveries that may lead to improved public health and health care. The Center focuses on areas that will have the greatest impact by prioritizing research topics that show scientific opportunity and promise and are amenable to rigorous scientific inquiry. As a responsible steward of its publicly provided resources, NCCIH is highly selective in promoting a broad range of highly meritorious basic and clinical research.
New analyses of 2012 National Health Interview Survey (NHIS) data indicate that about 59 million Americans spend approximately $30.2 billion out-of-pocket on complementary health approaches a year. This amount represents 9.2 percent of all out-of-pocket spending by Americans on health care and 1.1 percent of total health care spending. 1 Many of these individuals seek complementary health approaches to improve their health or to manage symptoms of chronic diseases or the side effects of conventional medicine.
Reducing Pain and Improving Symptom Management
NCCIH supports research to better understand pain and to identify effective complementary and integrative health approaches to reduce it. Pain is a major public health problem and is the most common reason Americans use these approaches. New analysis of the 2012 NHIS data reveals that more than half of American adults (125 million) have a musculoskeletal pain disorder, with 40 percent of these individuals using a complementary health approach. 2 In addition, the epidemic of prescription opioid misuse and overdose underscores the urgent need for safe and effective pain management. NCCIH scientists conducted a review of clinical trials, published in Mayo Clinic Proceedings, that suggests that popular mind and body approaches may be effective in managing common pain conditions—some showing more positive than negative results for certain types of pain. These include acupuncture and yoga for back pain, acupuncture and tai chi for osteoarthritis of the knee, massage therapy for neck pain, and relaxation techniques for severe headaches and migraine. Furthermore, a recently published NCCIH-supported randomized clinical trial compared mindfulness-based stress reduction (MBSR) with usual care (UC) and cognitive-behavioral therapy (CBT) and showed MBSR and CBT training, compared with UC, improved functioning and significantly reduced chronic low-back pain symptoms at six months, with continued benefit from MBSR at one year. 3
Applying lessons learned from leading the NIH Common Fund’s Health Care Systems Research Collaboratory, NCCIH is committed to the pragmatic study of nonpharmacologic strategies to reduce pain and reduce reliance on opioids. Building on collaborations with the Departments of Defense and Veterans Affairs, NCCIH and other NIH components plan to support a coordinating center and clinical research as part of a major program focusing on pragmatic pain management strategies for our military and veteran populations. This research has the potential to transform clinical decision making about which complementary health approaches work for which pain conditions and for whom and how these approaches are best integrated into health care systems.
To further leverage its investments in pain research, NCCIH participates in trans-NIH efforts such as the NIH BRAIN (Brain Research through Advancing Innovative Neurotechnologies) Initiative, which is accelerating the development and application of technologies to better understand how the brain works. Additionally, NCCIH is supporting the NIH Common Fund’s Stimulating Peripheral Activity to Relieve Conditions (SPARC) program.
NCCIH’s intramural research program studies how the nervous system perceives and modifies pain—exploring ways that expectations, emotion, environment, and genetics affect its perception. How genetic variants affect human touch sensations is one important area of exploration. Recently, NCCIH and National Institute of Neurological Disorders and Stroke intramural researchers discovered that the PIEZO2 gene controls specific aspects of touch and proprioception—awareness of one’s body in space. 4 Understanding the roles of PIEZO2 and other genes in these senses may provide clues to the processing of sensations such as pain.
Advancing Research on Natural Products
Americans spent $12.8 billion out-of-pocket on natural product supplements, such as fish oil/omega-3 fatty acids and probiotics, about one-quarter (24 percent) of what they spent out-of-pocket on prescription drugs ($54.1 billion). 1 To provide consumers and health care providers with the evidence to make informed decisions, NCCIH supports rigorous research on promising natural products. NCCIH identified areas of high priority for developing and pilot-testing natural products, emphasizing the public’s interest and use, and biologic plausibility for the product’s mechanisms of action and potential clinical benefit. In FY 2016, NCCIH released two funding opportunity announcements to solicit grant applications in these areas. NCCIH also is streamlining natural product clinical research using a phased-research pipeline starting with early phase studies exploring bioavailability or the extent to which a nutrient or medication can be used by the body, and the mechanism of action. Only the most promising natural products will advance to the later research phases that support investigations comparing clinical outcomes and biological effects through randomized controlled efficacy trials.
Inspiring Public Trust through Stewardship
To enhance its research investments, NCCIH released a new strategic plan in June 2016. With input from NIH staff, external stakeholders, and scientific advisors, the strategic plan addresses scientific gaps and opportunities under three scientific and two cross-cutting objectives. As the Center adapts to reduced funding, the NCCIH will continue to support its highest priorities as outlined in the Strategic Plan. The scientific objectives in the new plan are aligned with those of the NIH-Wide Strategic Plan.
Due to the self-care nature of many complementary health approaches, the dissemination of unbiased, evidence-based information is of great importance to the Center. NCCIH provides information to consumers, health care providers, and policymakers through multiple channels, including the Internet, broadcast and print media, a research blog, and a social media program.
Overall Budget Policy:
The FY 2018 President’s Budget request is $101.793 million, a decrease of $28.747 million compared with the FY 2017 Annualized CR level. These reductions are distributed across all programmatic areas and basic, epidemiology, or clinical research.
- Nahin RL, et al. Expenditures on complementary health approaches: United States, 2012. National Health Statistics Reports; no. 95, 2016.
- Clarke TC, et al. Use of complementary health approaches for musculoskeletal pain disorders among adults: Untied States, 2012. National Health Statistics Reports; no. 98, 2016.
- Cherkin DC, et al. Effects of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315(12):1240-1249.
- Chesler AT, et al. The role of PIEZO2 in human mechanosensation. N Engl J Med. 2016;375(14): 1355-1364.
Program Descriptions and Accomplishments
Extramural Clinical Research: The NCCIH extramural research program funds clinical investigations on complementary and integrative health practices and interventions. Projects range from small pilot studies to large-scale clinical trials and epidemiologic studies, including several collaborations between NIH ICs and other Federal agencies. For example, recent results from an NCCIH-supported study, published in the Journal of the American Medical Association, expand the evidence base for the effectiveness of mind and body interventions. In the first randomized clinical trial to rigorously evaluate mindfulness-based stress reduction (MBSR) for young and middle-aged adults with chronic low back pain, researchers compared MBSR with usual care (UC) and cognitive-behavioral therapy (CBT). They found that individuals with chronic low back pain who received training in MBSR and CBT, compared with UC, demonstrated greater improvements in functioning and significant reductions in chronic low back pain at six months with MBSR and CBT and continued benefit from MBSR one year following treatment. The persistence of these beneficial effects suggests that MBSR and CBT may provide patients with skills needed to maintain long-term pain relief.
Program Portrait: Assessing the Potential Cardiovascular Benefit of Chelation Therapy for Older Patients with Diabetes and Heart Disease
In FY 2016, NIH launched a replication trial to determine the potential cardiovascular benefits of chelation therapy for older patients with diabetes and heart disease. Chelation treatments involve intravenous (through the veins) infusion of a drug, ethylenediaminetetraacetic acid (EDTA). EDTA binds certain metals and minerals including lead, cadmium, and calcium, and causes their excretion from the body. Chelation is conventionally used as a treatment for heavy metal (e.g., lead) poisoning. While some people use chelation as a treatment for conditions like heart disease, its use to treat this condition is unproven and not approved by the U.S. Food and Drug Administration.
This new trial was designed to further investigate the results from the earlier NIH-funded Trial to Assess Chelation Therapy (TACT). In 2013, initial TACT findings published in the Journal of the American Medical Association showed that infusions of chelation therapy using EDTA produced a modest but statistically significant reduction in cardiovascular events in all EDTA-treated participants compared to patients who received placebo infusions. Further examination of the data, however, showed that patients with diabetes who received the active treatment had striking reductions in their risk for any cardiovascular events, death from heart disease, nonfatal stroke, or nonfatal heart attack, and a significant reduction in death from any cause. In contrast, there was no significant benefit of EDTA treatment in the roughly two-thirds of study participants who did not have diabetes.
While these results are intriguing, further research is needed to examine whether this treatment benefit can be replicated, a step needed to establish benefits of chelation in diabetics. To address this need, NIH is conducting a trial to test the primary hypothesis that chelation therapy in patients with diabetes and a prior myocardial infarction (heart attack) improves event-free survival. The new trial will recruit approximately 1,200 participants from more than 100 study sites. If the TACT results are replicated, their clinical significance would likely change the way patients with diabetes and a history of myocardial infarction would be treated in the future.
NCCIH, National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, and National Institute of Environmental Health Sciences are providing co-funding for this trial, which is known as TACT2.
Extramural Basic Research: Basic research on fundamental biological effects and active components of interventions is central to the development of the evidence base on complementary and integrative health approaches and underpins the design of clinical research. NCCIH supports investigator-initiated basic research and will continue, through targeted initiatives, to support basic and translational research on promising complementary interventions. Additionally, NCCIH is energizing the research community to overcome the methodologic and technologic obstacles hindering basic research on natural products. For example, NCCIH and the NIH Office of Dietary Supplements are funding two Centers for Advancing Natural Products Innovation and Technology. The Centers are developing pioneering methods and techniques to catalyze new research approaches and technologies that will have significant impact on the chemical and biological investigation of natural products.
Extramural Research Training and Capacity Building: Improving the capacity of the field to carry out rigorous research on complementary health interventions is a high priority for NCCIH. To increase the number, quality, and diversity of investigators who conduct research on complementary health approaches, NCCIH supports a variety of training and career development activities for pre- and postdoctoral students, researchers, and clinicians. As part of its strategic planning effort, a special Working Group of the National Advisory Council on Complementary and Integrative Health examined the topic of the complementary and integrative health research workforce. Their report, which focuses specifically on ways to expand and strengthen the clinician-scientist workforce, was presented and discussed at the February 2016 Council meeting. The report recommendations will help shape NCCIH’s future training and career development programs. An implementation strategy based on the recommendations from the report to enhance research training and increase the number of clinician-scientists with complementary and integrative health experience is being developed.
Intramural Research: NCCIH’s intramural research program is focused on understanding the central mechanisms of pain and its modulation, with the long-term goal of improving clinical management of chronic pain through the integration of pharmacologic and nonpharmacologic approaches. Among topics of interest are the pathways and mechanisms by which expectations, emotion, attention, and other such processes modulate pain or pain processing. The program both engages and leverages the exceptional basic and clinical research talent and resources of other neuroscience and neuroimaging efforts within the NIH intramural community.
Research Management and Support (RMS): Through its RMS activities, NCCIH provides administrative, budgetary, logistical, and scientific support in the review, award, monitoring, and management of research grants, training awards, and contracts. In addition, NCCIH provides objective and evidence-based information to the public, scientists, and health care providers so that they may make informed decisions about the use of complementary and integrative health practices.
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Detail of Full-Time Equivalent Employment (FTE)
| OFFICE/DIVISION | FY 2016 Actual | FY 2017 Est. | FY 2018 Est. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| 1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| Office of Scientific Review | |||||||||
| Direct: | 5 | - | 5 | 5 | - | 5 | 5 | - | 5 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 5 | - | 5 | 5 | - | 5 | 5 | - | 5 |
| Basic and Mechanistic Research in Complementary and Integravtive Health Branch | |||||||||
| Direct: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Clinical Research in Complementary and Integrative Health Branch | |||||||||
| Direct: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Division of Extramural Activities | |||||||||
| Direct: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 2 | - | 2 | 2 | - | 2 | 2 | - | 2 |
| Division of Extramural Research | |||||||||
| Direct: | 8 | 1 | 9 | 9 | 1 | 10 | 9 | 1 | 10 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 8 | 1 | 9 | 9 | 1 | 10 | 9 | 1 | 10 |
| Divison of Intarmural Research Program | |||||||||
| Direct: | 7 | 1 | 8 | 7 | 1 | 8 | 7 | 1 | 8 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 7 | 1 | 8 | 7 | 1 | 8 | 7 | 1 | 8 |
| Office of Administrative Operations | |||||||||
| Direct: | 15 | - | 15 | 15 | - | 15 | 15 | - | 15 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 15 | - | 15 | 15 | - | 15 | 15 | - | 15 |
| Office of Clinical and Regulatory Affairs | |||||||||
| Direct: | 3 | 1 | 4 | 3 | 1 | 4 | 3 | 1 | 4 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 3 | 1 | 4 | 3 | 1 | 4 | 3 | 1 | 4 |
| Office of Communications and Public Liaison | |||||||||
| Direct: | 10 | - | 10 | 10 | - | 10 | 10 | - | 10 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 10 | - | 10 | 10 | - | 10 | 10 | - | 10 |
| Office of Grants Management | |||||||||
| Direct: | 6 | - | 6 | 6 | - | 6 | 6 | - | 6 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 6 | - | 6 | 6 | - | 6 | 6 | - | 6 |
| Office of Policy, Planning and Evaluation | |||||||||
| Direct: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 4 | - | 4 | 4 | - | 4 | 4 | - | 4 |
| Office of the Director | |||||||||
| Direct: | 5 | - | 5 | 5 | - | 5 | 5 | - | 5 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 5 | - | 5 | 5 | - | 5 | 5 | - | 5 |
| Total1 | 69 | 3 | 72 | 70 | 3 | 73 | 70 | 3 | 73 |
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Detail of Full-Time Equivalent Employment (FTE) (continued)
| FISCAL YEAR | Average GS Grade |
|---|---|
| 2014 | 12.7 |
| 2015 | 12.6 |
| 2016 | 12.8 |
| 2017 | 12.8 |
| 2018 | 12.8 |
NATIONAL INSTITUTES OF HEALTH
National Center for Complementary and Integrative Health
Detail of Positions1
| GRADE | FY 2016 Final | FY 2017 Annualized CR | FY 2018 President’s Budget |
|---|---|---|---|
| 1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||
| Total, ES Positions | 0 | 0 | 0 |
| Total, ES Salary | 0 | 0 | 0 |
| GM/GS-15 | 9 | 9 | 9 |
| GM/GS-14 | 15 | 15 | 15 |
| GM/GS-13 | 17 | 18 | 18 |
| GS-12 | 8 | 8 | 8 |
| GS-11 | 3 | 3 | 3 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 1 | 1 | 1 |
| GS-8 | 1 | 1 | 1 |
| GS-7 | 3 | 3 | 3 |
| GS-6 | 0 | 0 | 0 |
| GS-5 | 0 | 0 | 0 |
| GS-4 | 0 | 0 | 0 |
| GS-3 | 0 | 0 | 0 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 57 | 58 | 58 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207) | 0 | 0 | 0 |
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 1 | 1 | 1 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 1 | 1 | 1 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 3 | 3 | 3 |
| Ungraded | 11 | 11 | 11 |
| Total permanent positions | 61 | 62 | 62 |
| Total positions, end of year | 71 | 72 | 72 |
| Total full-time equivalent (FTE) employment, end of year | 72 | 73 | 73 |
| Average ES salary | 0 | 0 | 0 |
| Average GM/GS grade | 12.8 | 12.8 | 12.8 |
| Average GM/GS salary | 113,700 | 115,974 | 115,974 |