Product Integrity Policy Sample Response
Note: This document provides an example for researchers to follow when responding to the NCCIH Product Integrity Policy. This example includes the preferred amount of data and level of detail expected for characterization of a complex natural product mixture. Some information has been edited for sharing online.
Principal investigator: Amala Soumyanath, Ph.D.
Re: __________
Product Information: _________
A. Complex Botanical Products
(e.g., plants, macroscopic fungi, algae)
For single-herb products, information on the raw material and final product is needed.
The raw material is dried Centella asiatica (CA) herb. We will be making our own extracts and fractions, and isolating single compounds from CA. We will also use single chemical reference compounds purchased from commercial sources.
For multiple-herb products, information is needed for each individual botanical within the preparation, as well as for the mixture as a whole.
Not applicable. We will be working with a single herb, Centella asiatica (CA).
In vitro studies
1. Identify the source plant for the product using the scientific taxonomic nomenclature and author citation (e.g., Ginkgo biloba L.). Describe the parts of the plant from which the product is derived.
Source plant: Centella asiatica (L.) Urban, Family Apiaceae. (CA).
We will use dried CA whole herb aerial parts (i.e., leaves and stems with ≤5% roots).
2. If an extract is used, identify the extraction solvent.
The primary focus of our study will be a water extract of CA (CAW). We will also generate fractions and isolate single components from this extract.
In addition, we expect to perform some comparative studies using extracts of CA made with ethanol and other solvents. The range of solvents used for extraction, fractionation and compound isolation may include hexane, ethylacetate, dichloromethane, chloroform, propanol, isopropanol, methanol, acetone, acetonitrile, and pH modifiers including formic acid, acetic acid, ammonia or diethylamine. We will include all pertinent details of the extraction, fractionation and isolation methods in publications to allow replication of the work.
3. Name the supplier of the product. Include the brand name of the product, if applicable.
Dried CA whole herb (aerial parts and ≤5% roots) will be purchased from AAA Herb Company in a large quantity (10kg) sufficient for the entire study. The herb is sold under the commercial name “gotu kola.” There is no brand name other than that of the supplier, AAA Herb Company.
4. Present data on the characterization (e.g., chemical profile or fingerprint) of the final product as thoroughly as the state of the science allows.
- Identify active and/or other relevant marker compound(s) used for standardization.
- Provide any information relevant to the standardization process (including process control, as well as chemical and/or biological standardization of ingredients).
- Information from suppliers MUST be confirmed through independent analysis either by a third party lab or by the investigator (if they have appropriate facilities and expertise).
Supplier: CA will be purchased from AAA in large quantity (10kg) to ensure the same material can be used for the entire study.
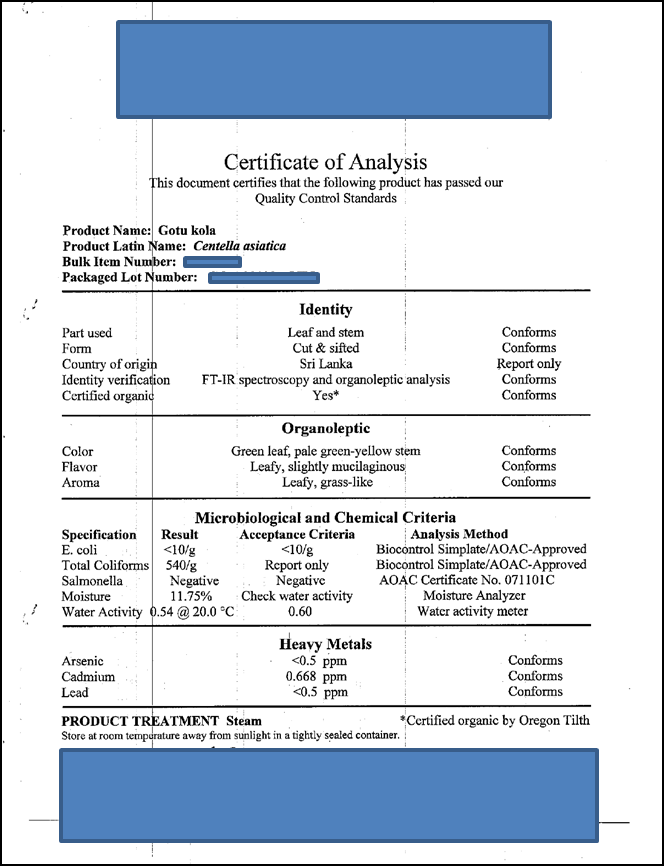
Identification: AAA will perform preliminary identification of the CA sample using in-house organoleptic tests, macroscopic examination, and Fourier Transform Infra-Red (FTIR) spectroscopy, an established chemometric method for the identification of botanical powders (1,2). The FTIR spectrum obtained will be matched by computer, to a database of composite spectra from historical, verified CA samples. Identity is considered verified for a minimum match value of 50, and occurrence of the expected herb name in top 10 closest matches. AAA will provide a certificate of analysis (see Appendix 2) with identity and impurity test results, and geographical source information.
Independent verification of herb identity will be performed by the PI and collaborators at OHSU and OSU, using in-house thin layer chromatography (TLC) methods and liquid chromatography coupled to ultraviolet spectroscopy or mass spectrometry (LC-UV-MS) as previously described by us (3,4,5). Excerpts from our publications are given in Appendix 3. Both low/medium resolution mass spectrometry and high resolution mass spectrometry (for accurate mass analysis) will be utilized as appropriate. Using these methods, we will verify the presence of characteristic CA triterpenes (asiatic acid, madecassic acid, asiaticoside and madecasoside), and caffeoylquinic acids (3-, 4-, or 5- monocaffeoylquinic acids and 1,3-, 1,5- , 3,5-, 4,5- and 3,4- dicaffeoylquinic acids) as shown in Appendix 4. This will be achieved by comparison of water and ethanol extracts of CA, against co-chromatographed commercial reference standards (Company B or Company C). We will document the chemical fingerprint (TLC and LC-UV-MS) of small scale water and ethanol extracts made for the purpose of identification of the initial herb sample.
Standardization: After confirming the neuroprotective activity of a pilot CAW extract in MC65 cells (our primary bioactivity assay) as in our earlier studies (3,5), we will obtain a large quantity of this batch of plant material (10kg) for use throughout our study and characterize this batch in terms of the content of the triterpenes and caffeoylquinic acids using TLC and LC-UV-MS (3,4,5). CA is grown in several tropical countries but is mainly sourced from India, Sri Lanka, and Madagascar. If we need to use further batches of CA, we will attempt to obtain a batch from the same original geographical source. We will quantify the triterpenes and caffeoylquinic acids, and confirm neuroprotection of MC65 cells in a small sample of new CA batches. The material will only be purchased if it shows neuroprotective in MC65 cells and is within ±20% of the original batch with respect to the content of triterpenes and caffeoylquinic acids.
For in vitro and in vivo studies, we will prepare a total of 500g of CAW by means of several extractions from the same batch of CA, within 6 months of its purchase. CAW will be prepared by reflux extraction (6) with double distilled water as in our earlier studies (3,5), and freeze-dried. We will carefully follow a standard extraction protocol (controlling herb: water ratio, time and temperature) to minimize variation between extracts (see Appendix 5). Each CAW extract will receive a unique identifier, and will be fingerprinted by TLC and LC-UV-MS. Voucher samples of each extract (1g) will be stored. CAW extracts showing >10% difference in the % weight extracted and in the % content of any major components from earlier batches will not be used in the study. During bioassay-guided fractionation, all major active fractions will be fingerprinted as outlined above for CAW. We will include all pertinent details of the extraction, fractionation, and isolation methods in publications to allow replication.
Documentation of product “fingerprints”: For documentation purposes, TLC profiles will be photographed under short and long wave UV light, and after spraying with anisaldehyde reagent (7). The CA triterpenes and their glycosides, as well as mono and and dicaffeoylquinic acids will be quantified by HPLC using commercial reference standards. For unknown compounds, we will document HPLC retention time, peak area, normalized % area, UV and mass spectra.
Voucher specimens: A voucher specimen (100g) of each batch of CA dried herbal material used in this project, along with appropriate source documentation, will be maintained in the PI’s laboratory. A second specimen (100g) will be deposited at the OSU herbarium (bpp.oregonstate.edu/herbarium). Voucher samples of principal extracts (1g) and active fractions (10–100mg) will be given unique identifiers and maintained at -20°C in the PI’s laboratory.
5. Provide a Certificate of Analysis from the supplier to show compliance to their specifications for content and any other supporting information relating to the batches to be used in the study. This should include data on the analysis of the product for contaminants/impurities such as pesticides, heavy metals, and residual solvents.
AAA will provide a Certificate of Analysis with identity and impurity test results, and geographical source information. We have not yet purchased the batch to be used in our study, and will obtain the relevant Certificate of Analysis only when we do so. However, a sample Certificate of Analysis issued by AAA for CA (gotu kola) is shown in Appendix 2. Impurity testing (E.coli, Salmonella, moisture content, lead, cadmium, arsenic) will be performed at AAA. AAA does not routinely test for pesticides, but will obtain pesticide analysis data for our study sample by contract to QQQ Labs.
6. Provide data on batch-to-batch reproducibility. These data may be required separately for the botanical alone, the vehicle alone, and the final product, as appropriate.
Variation in content of triterpenes and caffeoylquinic acids has been reported between different accessions of CA herb even within a relatively small geographical region (8,9). In our own studies, we have compared the content of triterpenes and caffeoylquinic acids in water extracts made from two different batches of CA, and compared the LC-UV profiles of multiple batches of CAW made from a single batch of CA. The data is given in Appendix 5. We have observed significant differences in the content of the triterpenes between the two different batches of CA. With multiple water extracts of the same batch of CA, we observed differences in the total amount of caffeoylquinic acids extracted although the relative proportions were less variable. Since we had standardized the herb:water ratio and the boiling time, differences appear to have arisen due to variable time allowed for cooling before filtration. For the proposed project, we will maintain much tighter control on the entire process of extraction, cooling and filtration to limit this variability by following a strict protocol.
7. Present a plan to monitor the stability of product samples from all batches used during the course of the study. The plan should include:
- How you plan to store the botanical, vehicle, and/or final product
- What analyses will be conducted, the methods to be used, and how frequently and by whom the analyses will be done
- Investigator's tolerances for chemical and/or biological variability, and what will be done if variability exceeds those limits.
In addition, sufficient material must be retained from each batch to allow independent analysis, should NCCIH request samples. Samples should be retained a minimum of one year after all results arising from the study have been published.
Storage: CA plant material will be stored in the dark at room temperature. We intend to complete all extractions required to obtain 500g of CAW within 6 months of purchase of the plant material. Extracts will be dried and stored in a freezer at -20°C. Solutions of extracts will also be stored at -20°C.
Stability testing: As mentioned earlier, we plan to complete our extraction of CA plant material within 6 months of purchase. If we use plant material stored for longer than 6 months, stability will be determined by comparing LC-MS-UV fingerprints of freshly prepared water and ethanol extracts to frozen extracts prepared previously from that batch of CA using the same extraction method.
We have found that the water extract, CAW, is stable when stored at -20°C. An extract stored at -20°C in dried form for 5 years showed the same effect on MC65 cells, as when originally tested. Nevertheless, all extracts will be characterized by LC-UV-MS at the time of preparation. If used at greater than 6 months from preparation, the extracts will be re-analyzed and their fingerprint compared to the original chromatogram.
Tolerances: When a new batch of CAW is made, it will be used only if the content of triterpenes and caffeoylquinic acids is within ±10% of the mean value of all previous batches. If a previously prepared sample of CAW is to be used at >6 months from preparation, it will be analyzed by LC-UV-MS and used only if its content of any individual triterpene or caffeoylquinic acid is within 10% of its original value when freshly made. The value of ±10% is based on standard pharmaceutical tolerances for mixtures.
Contingency for variability: If new CA plant material is to be used, an initial sample will be analyzed to determine if it is close to the original sample in chemical profile and biological activity in MC65 cells (5). However, if it is not possible to find a batch which lies within the chemical limits (±20%) proposed earlier, then the study will proceed with the new botanical sample, provided activity in MC65 cells is maintained. We will ensure that we characterize, document, and report differences from the original batch.
For CAW extracts, if limiting variability to (±10%) proves difficult, we will use each CAW extract at an equivalent concentration of caffeoylquinic acids, which appear to be the neuroprotective actives in CA (3,5).
Voucher samples: We will store voucher specimens of all batches of CA used (2 x 100g), all major extracts (1g) and major active fractions (10–100mg). Extracts and fractions will be stored in dried form at -20°C as previously described under Item 4 above.
Additional Information for Animal Studies
8. Describe the method of authentication of the raw material and where and how an authenticated voucher specimens of the plant material is reserved. Ideally, an original herbarium specimen should be deposited in a publicly accessible herbarium where conditions and staff are appropriate for the conservation of the specimen. At a minimum, a voucher or reference sample culled from the same batch of the plant material should be deposited in a secure, climate-controlled facility at the investigator's institution.
Authentication, characterization, and deposition of voucher specimens will be performed as described under points 4 and 7 above for in vitro studies.
9. Describe the manufacturing process. This should:
- Include details of the extraction such as solvent(s), ratio of plant to solvent, time and temperature
- Alternatively, if the test agent is not an extract, but is an infusion, tincture, or volatile oil, describe its preparation with the same level of detail as an extract
- Provide information about the formulation of the final product (e.g., excipients, chow/feed composition, etc.)
- Provide documentation to show the product was manufactured under GMP guidelines.
Extraction method: These studies will use a water extract of CA (CAW) prepared, standardized and characterized as described in Item 4 (in vitro studies) above.
Formulation: The extract (CAW) will be dissolved in the drinking water of animals; no excipients will be used.
GMP guidelines: AAA, the supplier of CA herb, operates under GMP guidelines. The CAW extract to be used in our studies will be prepared in house, in a laboratory at OHSU and not under GMP guidelines. This extract will not be used in any human studies but will be sufficiently well characterized (see Item 4, above) to allow a similar extract to be made in a GMP compliant laboratory in any future human studies.
10. If the product is combined with a diet, a Certificate of Analysis and specifications for the vehicle will also be necessary to assure purity, consistency, absence of bioactive components (e.g., soy), and/or reproducibility of the vehicle. Furthermore, analysis of the formulated diet may be required to assess consistency, stability, etc. of the product in this matrix.
For our studies the extract (CAW) will be dissolved in double distilled water and used in place of drinking water for the test animals. For in vivo studies, we will determine the stability of CAW in drinking water to determine the required frequency of providing fresh solutions.
D. Refined Products
(e.g., pure compounds, highly enriched extracts, prebiotics, vitamins, minerals, amino acids)
In Vitro Studies
1. Name the supplier of the product.
Our study will examine the effects of single compounds in vitro and in vivo. These compounds will be either
- single compounds isolated from CA in the course of our studies.
- commercially available compounds known to occur in CA.
Sources:
| Supplier | Compounds to be purchased (structures of all compounds are given in Appendix 4) |
|---|---|
| Company B | Triterpenes: asiatic acid, Caffeoylquinic acids: chlorogenic acid, neochlorgenic acid, 4-O-caffeoylquinic acid (cryptochlorogenic acid), 1,5-dicaffeoyquinic acid. |
| Company C | Triterpenes: madecassic acid, madecassoside, asiaticoside, Caffeoylquinic acids: 1,3-dicaffeoylquinic acid, isochlorogenic acid A, isochlorogenic acid B, isochlorogenic acid C. |
2. Present data on the characterization of the product (e.g., chemical profile or fingerprint) as thoroughly as the state of the science allows.
All commercial compounds will be examined in house by LC-UV-MS in order to confirm that their retention times, UV spectra and MS are as expected. The small quantities and high cost of these compounds precludes their in-house analysis by the more selective technique of NMR spectroscopy.
Provide data on the analysis of the product for contaminants/impurities such as pesticides, heavy metals, and residual solvents.
The certificates of analysis of the commercial compounds have a loss on drying, or % moisture content but do not list pesticides, heavy metals, or residual solvents.
3. Provide data on batch-to-batch reproducibility. These data may be required separately for the agent alone, the vehicle alone, and the final product, as appropriate.
Not applicable.
4. Present a plan to monitor stability of product samples from all batches used during the course of the study. The plan should include:
Additional Information for Animal Studies
6. Provide information relevant to the manufacturing process assuring reasonably consistent material suitable for scientific study (including process control, as well as chemical standardization of ingredients).
For in vivo experiments compounds will be dissolved in drinking water if sufficient quantities are available. Alternatively, an aqueous (saline) solution will be prepared for administration by gavage. As compounds are derived from an aqueous extract (CAW), we expect they will be soluble in water.
Commercial compounds from Company B and Company C should be prepared under GMP guidelines, whereas compounds isolated in our laboratory will not fall under these formal guidelines.
7. When the product is combined with a diet, a Certificate of Analysis and specifications for the vehicle will also be necessary to assure purity, consistency, absence of bioactive components and/or reproducibility of the vehicle. Furthermore, analysis of the formulated diet may be required to assess consistency, stability, etc., of the product in this matrix.
We will administer these compounds in aqueous solutions—either in drinking water or by gavage. Stability in water will be determined in a preliminary study using LC-UV-MS and fresh solutions will be made up accordingly during long-term studies.
Appendix 1—References cited
Appendix 2: Sample Certificate of Analysis of CA herb from AAA (sold as Gotu kola).
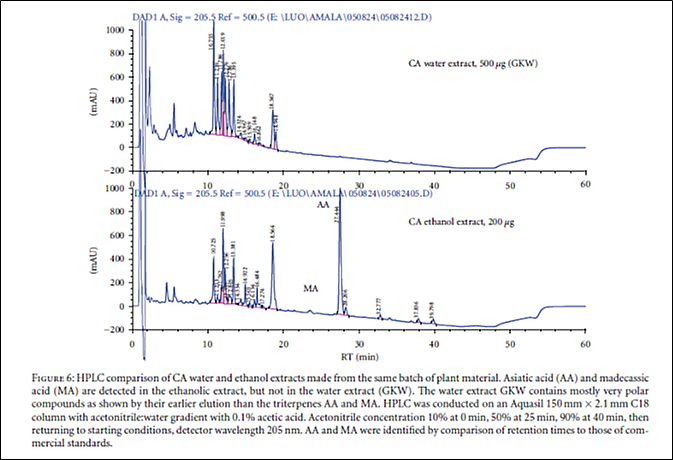
Appendix 3: Examples of our published in-house HPLC analyses of CA compounds
Comparison of CA water and ethanol extracts showing peaks including the major triterpenes (From (3))
Comparison of CAW and reference caffeoylquinic acids (From (5))
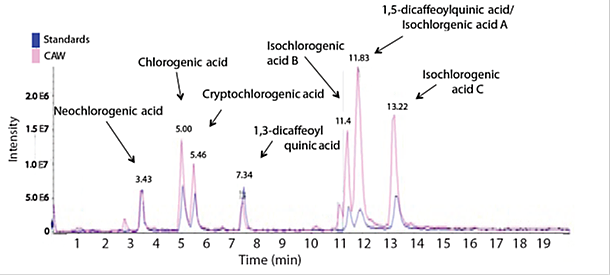
LC-MS was performed on an Applied Biosystems 4000 Q-trap using a Shimadzu LC pump with an Agilent Extend C18 column (2.1×150mm, 5_m) eluting with a gradient of acetonitrile in water both with 0.1% formic acid acetonitrile 5 to 18% in 9min, up to 25% at 20min, then to 95% at 22min, returning to 5% at 22.5min, and maintained there until 30min).MS analysis was performed in negative ion mode, with a source temperature of 450◦250 C, and source voltage of 4.5 kV. Mass spectra were acquired utilizing enhanced product ion (EPI) mode in which two experiments were used, the first selecting ions with m/z 515 and the other 353, and collisionally induced dissociation was performed using a collision energy of –30V. LC-MS was performed on an Applied Biosystems 4000 Q-trap using a Shimadzu LC pump with an Agilent Extend C18 column (2.1×150mm, 5_m) eluting with a gradient of acetonitrile in water both with 0.1% formic acid (acetonitrile 5 to 18% in 9min, up to 25% at 20min, then to 95% at 22min, returning to 5% at 22.5min, and maintained there until 30min).
Appendix 4: Characteristic compounds found in Centella asiatica
Triterpenes
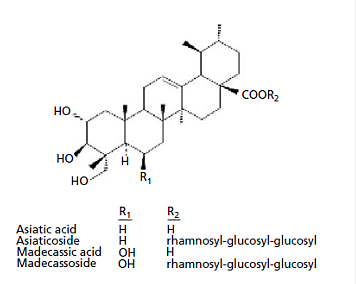
Caffeoylquinic acids
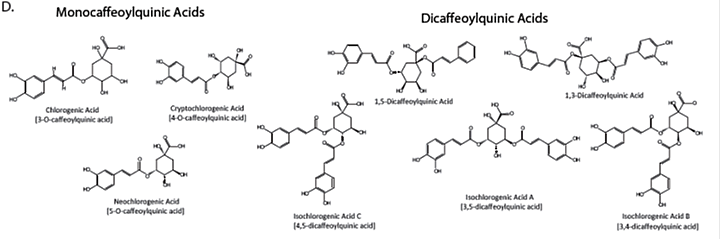
Appendix 5: Batch to Batch variability
A. Comparison of the composition of water extracts of CA made from two different batches of plant material using the same extraction method.
GKW was a water extract of CA Batch no. ABCD; AAA Herb Company.
CAW1 was a water extract of CA Lot 1234, BBB Botanical Company.
The relative content of triterpenes and caffeoylquinic acids as determined by LC-UV in GKW and CAW1 is given in the table below:
| Extract → | CAW 1 (% w/w) | GKW (%w/w) |
|---|---|---|
| Compound ↓ | ||
| Chlorogenic acid | 0.107 | 0.095 |
| Neochlorogenic acid | 0.051 | 0.013 |
| Cryptochlorogenic acid | 0.007 | 0.011 |
| 1,5-dicaffeoylquinic acid / Isochlorogenic acid A (unresolved) | 0.674 | 0.907 |
| 1,3 dicaffeoyquinic acid | 0.039 | 0.025 |
| Isochlorogenic acid B | 0.287 | 0.276 |
| Isochlorogenic acid C | 0.278 | 0.256 |
| Asiatic acid | nd | nd |
| Madecassic acid | nd | nd |
| Asiaticoside | 0.944 | 0.228 |
| Madecassoside | nd | Nd |
nd = not detected; caffeoylquinic acids were detected at 330nm; triterpenes at 205nm.
B. Comparison of the composition of multiple water extracts made from the same batch of plant material using the same method.
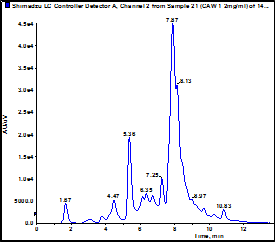
The figure shows an LC-UV trace of CAW 1 performed on an Agilent ZORBAX SB –C18; 150mm x 2.1mm; 1.8µ with guard run at 40C with an acetonitrile:1% formic acid gradient. Peaks were detected at 330nm. The table below compares peak areas for the major peaks in 3 different extracts made from the same batch of CA: Lot 1234, BBB Botanical Company. (The peak at 1.67 is due to solvent).
Conclusion: Each extraction gave different absolute amounts of the major components. However, the relative areas were similar in all extracts. This is believed to be due to variations in processing/cooling time between boiling and removal of plant material by filtration leading to differences in overall contact time and hence extents of extraction. This factor will be controlled in our new study.
| Peak RT | Peak area / 10,000 | Peak area relative to 5.4 min peak | ||||
|---|---|---|---|---|---|---|
| CAW 1 | CAW 2 | CAW 3 | CAW 1 | CAW 2 | CAW 3 | |
| 4.5 | 10.4 | 57.9 | 39.4 | 0.40 | 0.32 | 0.44 |
| 5.4 | 25.9 | 182.2 | 90.4 | 1.00 | 1.00 | 1.00 |
| 6.1 | 3.7 | 24.5 | 10.7 | 0.14 | 0.13 | 0.12 |
| 6.4 | 4.7 | 35.7 | 14.6 | 0.18 | 0.20 | 0.16 |
| 6.7 | 3.8 | 28.7 | 12.7 | 0.15 | 0.16 | 0.14 |
| 7.25 | 6.7 | 48.3 | 23.3 | 0.26 | 0.27 | 0.26 |
| 7.5-8.8 | 128 | 964.3 | 484.7 | 4.94 | 5.29 | 5.36 |
| 8.97 | 0.4 | 3 | 1.6 | 0.02 | 0.02 | 0.02 |
- Isolated compounds will be obtained through fractionation and purification of CAW using standard methods of phytochemical isolation, principally preparative chromatography (6).
- For compounds isolated in our project, we will confirm or elucidate their structures using standard spectroscopic techniques, in particular accurate mass high resolution mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy. Our collaborators at OSU will be performing the isolation and have access to state of the art equipment for fractionation of extracts, as well as the appropriate MS and NMR instrumentation (see resources page).
- For the commercial products, Company B provides batch specific certificates of analysis showing mass spectra, NMR spectra and HPLC traces for all their chemical standards along with the compounds. Company C has batch-specific certificates of analysis available online. We will inspect these for compliance.
- Indicate the purity if the product is a single component.
All commercial compounds are stated to be ≥95% pure by HPLC analysis. We will attempt to purify our isolated compounds to this level of purity. - Provide concentration of major and minor components if the product is a mixture.
not applicable - Information from suppliers MUST be confirmed through independent analysis either by a third party lab or by the investigator (if they have appropriate facilities and expertise).
All compounds isolated by us from CA will undergo detailed spectroscopic characterization and structure elucidation as described under section 2a above. - How you plan to store the test agent, vehicle, and/or final product
All commercial chemical standards will be stored in accordance with supplier’s recommendations when in solid form. All compounds isolated from CA in our studies will be stored at -20°C. Solutions of any of these compounds will routinely be stored at -20°C in the freezer. - What analyses will be conducted, the methods to be used, and how frequently and by whom the analyses will be done
We will analyze the compounds using LC-UV-MS methods (3,5). Stability will be evaluated every time the sample is to be used as a chromatographic standard or in an in vitro or in vivo study. Chromatograms will be examined for evidence of decomposition e.g. significantly smaller or larger peak than previously found, presence of additional peaks or altered UV or MS spectral characteristics. - Investigator’s tolerances for chemical or biological variability and what will be done if variability exceeds those limits. In addition, sufficient material must be retained from each batch to allow independent analysis, should NCCIH request samples. Samples should be retained a minimum of 1 year after all results arising from the study have been published.
To minimize the effects of batch to batch variability, we will only use commercial compounds that are ≥95% pure. Although standard pharmaceutical practice is to require 100% ±2% purity for single chemical drugs, we are allowing a wider tolerance to account for the difficulty in purifying phytochemicals from closely related substances present in the extract. In the case of compounds isolated by us from CAW, we will attempt to adhere to the ≥95% pure standard. However, purification to this standard may prove difficult due to either the presence of very closely related compounds, or having too small quantities of the isolated substance for further purification. In this situation, we will proceed to use the substance in our study, but record the purity level and any other data characterizing the nature of the mixture. This information will be reported in any publications. - Provide information on the formulation of the final product (e.g., excipients, solvents, buffers, chow/feed composition).
- Provide documentation to show product was manufactured under GMP guidelines.
- Bunaciu AA, Aboul-Enein HY, Fleschin S. Recent applications of fourier transform infrared spectrophotometry in herbal medicine analysis. Applied Spectroscopy Reviews. 2011;46(4):251–260.
- Gad HA, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: A review. Phytochem Anal. 2013;24(1):1–24.
- Soumyanath A, Zhong Y-, Henson E, Wadsworth T, Bishop J, Gold BG, et al. Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer's disease: Investigation of a possible mechanism of action. Int J Alzheimer's Dis. 2012.
- Soumyanath A, Zhong YP, Gold SA, Yu X, Koop DR, Bourdette D, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J Pharm Pharmacol. 2005 Sep;57(9):1221–1229.
- Gray NE, Morré J, Kelley J, Maier C, Stevens JF, Quinn JF, Soumyanath A. Caffeoylquinic acids in Centella asiatica protect against β-amyloid toxicity. J Alzheimer’s Dis. 2014;40(2):359–373.
- Houghton PJ, Raman A. Handbook for the fractionation of natural extracts. London: Chapman and Hall; 1998.
- Wagner Hbs. Plant drug analysis. A thin-layer chromatographic atlas. Berlin: Springer-Verlag; 1996.
- Long HS, Stander MA, Van Wyk B. Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. S Afr J Bot. 2012;82:53–59.
- Upadhyaya S, Saikia LR. Evaluation of phytochemicals, antioxidant activity and nutrient content of Centella asiatica (L.) urban leaves from different localities of Assam. Intl J Pharma Bio Sci. 2012;3(4):656–663.