New Funding Available for Basic and Mechanistic Research on Pain and Complementary Approaches: Explore NIH HEAL Opportunities
January 23, 2019
Are you interested in taking advantage of the NIH HEAL InitiativeSM funding opportunity announcements (FOAs) to conduct basic and mechanistic research to explore and discover new pain treatment options? The National Center for Complementary and Integrative Health (NCCIH) offers several FOAs for you to consider.
Investigators interested in the basic science of a natural product or a mind-and-body approach: How does my intervention work to achieve safe and effective pain treatment?
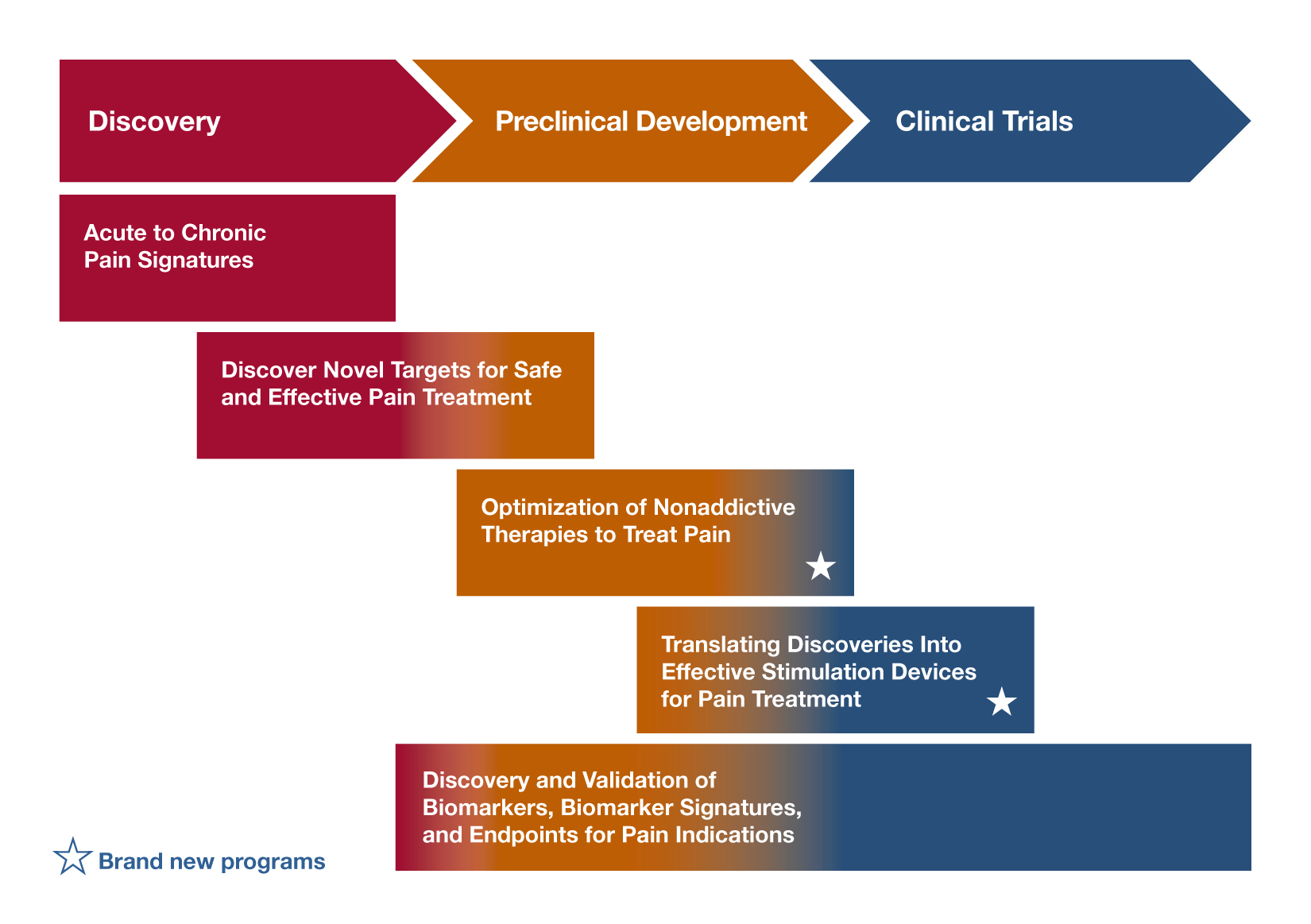
Discovery and Validation of Novel Targets for Safe and Effective Pain Treatment (R21; R01 Clinical Trial Not Allowed) – RFA-NS-18-042; RFA-NS-18-043
RFA-NS-18-042 encourages new research to discover and validate novel targets for pain treatment (for example, using an existing methodology to explore an area of basic biology that could uncover a novel pain treatment target). RFA-NS-18-043 focuses on the basic science discovery of targets in the peripheral nervous system, central nervous system, immune system or other body tissues that could help researchers develop treatments with minimal side effects and little to no abuse/addiction liability. If you’re interested in identifying the targets of a complementary therapy, to achieve analgesic effects, consider applying to one of these RFAs. Projects to identify novel pain targets (including acute/chronic pain, osteoarthritis, orofacial pain, and others) will be considered (as will those targeting overlapping chronic pain conditions). Researchers proposing projects identifying novel targets in specific populations also may respond to this FOA.
Contact: Inna Belfer, M.D., Ph.D.
Investigators interested in natural product and pain: How to make my therapies work better?
HEAL Initiative: Optimization of Non-addictive Therapies [Small Molecules and Biologics] to Treat Pain - (U44 Clinical Trial Not Allowed) – RFA-NS-19-020
Optimization of Non-addictive Therapies [Small Molecules and Biologics] to Treat Pain (UG3/UH3 Clinical Trial Not Allowed) – RFA-NS-19-010
Are you researching natural products and looking to optimize them for non-addictive analgesic properties through preclinical studies, consider this FOA. It supports preclinical optimization and development of safe, effective, and non-addictive small molecule and biologic therapeutics to accelerate optimizing/developing promising small molecule and biological hits/leads toward clinical trials. Applicants must have a promising hit/lead, robust biological rationale for the intended approach, and identified assays for optimizing the agent. The program includes optimization/early development activities, IND-enabling studies, and assembly of Investigational New Drug (IND) application(s).
Contact: Yisong Wang, Ph.D.
Investigators interested in device-based complementary therapies: How do I develop and translate the devices and eventually apply them for clinical usage?
HEAL Initiative: Translational Devices to Treat Pain (UG3/UH3 Clinical Trial Optional) – RFA-NS-19-016
Interested in developing a device to facilitate or enhance the delivery of natural products or mind and body approaches? Then consider applying to this FOA, which encourages investigators to pursue translational activities and clinical trials to treat pain with innovative, targeted, and non-addictive diagnostic and/or therapeutic devices (that avoid the need for opioids). The clinical trial should provide information about the device’s design and intended function that cannot be practically obtained through additional nonclinical assessments (e.g., bench top or animal studies) due to its novelty or intended use. NCCIH is interested in such translational devices that incorporate or enhance complementary and integrative health approaches for pain treatment, including natural products, probiotics, acupuncture and other manual therapies, meditation and meditative movement therapies, music interventions, or other art therapies.
Contact: Wen G. Chen, Ph.D.
HEAL Initiative: Translational Devices to Treat Pain (U44 Clinical Trial Optional) – RFA-NS-19-017
If your research interests involve device development, consider applying to this FOA. It encourages small business concerns (SBCs) to pursue translational activities and clinical trials to treat pain with innovative, targeted, and non-addictive diagnostic and/or therapeutic devices that avoid opioids. The clinical trial should provide information about the device function or final design that cannot be practically obtained through additional nonclinical assessments (e.g., bench top or animal studies) due to the novelty of the device or its intended use. NCCIH is interested in such translational devices that incorporate or enhance complementary and integrative health approaches for pain treatment, including natural products, probiotics, acupuncture and other manual therapies, meditation and meditative movement therapies, music interventions, or other art therapies.
Contact: Wen G. Chen, Ph.D.
HEAL Initiative: Clinical Devices to Treat Pain (UH3 Clinical Trial Optional) – RFA-NS-19-018
Are you developing a device to facilitate delivery of natural products or enhance other mind and body approaches? Then consider applying to this FOA. It encourages investigators to design/conduct a small clinical trial to obtain critical information with innovative, targeted, and non-addictive diagnostic and/or therapeutic devices that improve patient outcomes and decrease or eliminate the need to prescribe opioids. The clinical trial should provide data to answer key questions about the proposed device’s function or final design and should provide information that cannot be practically obtained through additional non-clinical assessments (e.g., bench top or animal studies) due to its novelty or its intended use. NCCIH is interested in such translational devices that incorporate or enhance complementary and integrative health approaches for pain treatment, including natural products, probiotics, acupuncture and other manual therapies, meditation and meditative movement therapies, music interventions, or other art therapies.
Contact: Wen G. Chen, PhD
Investigators interested in biomarkers for complementary therapies: What are good biomarkers for my therapies?
Discovery of Biomarkers, Biomarker Signatures, and Endpoints for Pain (R61/R33 Clinical Trial Optional) – RFA-NS-18-041
Analytical and/or Clinical Validation of a Candidate Biomarker for Pain (R61/R33 Clinical Trial Optional) – RFA-NS-18-046
If you’re interested in discovering or validating biomarkers to predict treatment responses for a complementary therapy, consider these FOAs. The focus of the first is to identify and validate pain biomarkers, biomarker signatures, and/or endpoints. Although research supported by this FOA can include animal studies, it must include preliminary human validation using carefully standardized human samples or human clinical studies. The goal of this initiative is to deliver candidate biomarkers, biomarker signatures, and/or endpoints that are ready for advanced clinical and analytical validation research.
You may want to consider RFA-NS-18-046, if 1) a candidate biomarker has already been identified, 2) assay technology has already been developed, and 3) a working hypothesis regarding Context of Use is in place. Research supported by this FOA will demonstrate that biomarker or endpoint change is reliably correlated with variables such as clinical outcome, pathophysiologic subsets of pain, therapeutic target engagement or response to a pain therapeutic; in addition, biomarker response will demonstrate specificity to the pain condition or therapeutic as demonstrated at multiple clinical sites. The goal of this FOA is to facilitate the advancement of robust and reliable biomarkers, biomarker signatures and endpoints of pain to application in clinical trials (Phase II clinical trials and beyond) and in the spectrum of clinical practice.
Contact: Wen G. Chen, Ph.D.
Summary
We hope this blog helps researchers differentiate between several HEAL Initiative opportunities for basic and mechanistic research on pain treatment. Investigators should read the FOAs for more information.
View NCCIH’s Role in the NIH HEAL Initiative to see a full list of FOAs that we are supporting.

Comments
Comments are now closed for this post.