Interacting With Your Scientific Review Officer
November 5, 2015
Who are scientific review officers (SROs) and what do they do?
SROs are scientists, most are former faculty members and NIH grantees, who manage the first level of peer review, commonly known as the study section. SROs are the people who take the first thorough look at your application, determine the expertise required for the review, recruit the external scientists to match that expertise, manage the study section meeting where the applications are discussed and scored, and prepare the summary statement for your application.
SROs uphold the core values of peer review at NIH at all points in the peer review process. These values are expert assessment, impartiality, transparency, fairness, confidentiality, integrity and efficiency.
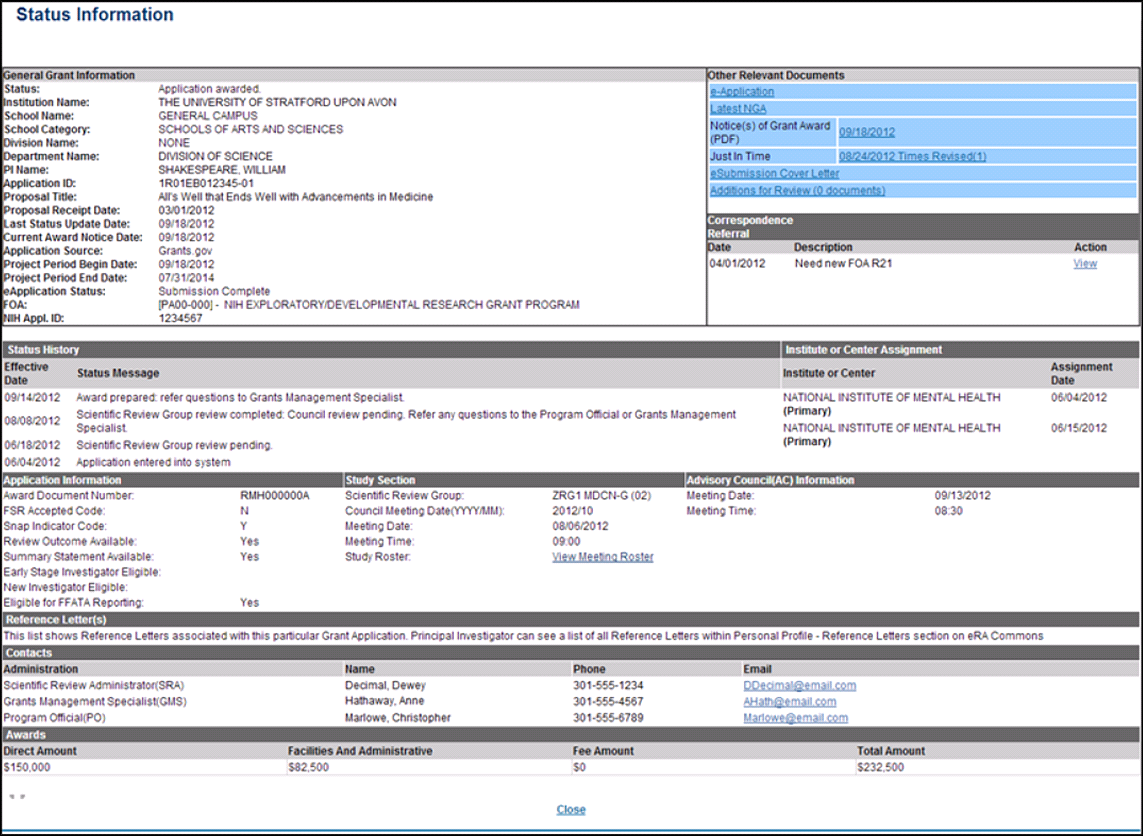
Who is your SRO and how do you reach them?
This information is available on the NIH Commons status page related to your application. It usually takes a few weeks for your application to be assigned to a specific SRO. The Commons status page will also list the assigned study section and when it will be meeting. See the Commons User guide, section 7 for instructions about accessing the status module.
When should you contact your SRO?
- If you have questions about the study section to which your application is assigned, contact the SRO to discuss it. The SRO cannot unilaterally change a study section assignment but can explain how you can make such a request, if warranted.
- If you want to suggest specific expertise needed for the review of your application, contact the SRO. Never suggest specific people; to do so would create a conflict of interest for those people and your application, which would prevent them from participating in the review.
- If you want to request that specific people be considered in conflict with your application, contact the SRO. Be prepared to explain the reasons. SROs make their own independent assessments to manage conflicts of interest, but will certainly take your opinions into consideration.
- If you want to submit additional material for your application, first read the NIH post-submission policy. Limited information can be submitted. If your additional material meets those requirements, contact the SRO to arrange submission and addition of the material to your application. All post-submission materials must be submitted no later than 30 days prior to the review meeting.
Don’t wait to contact the SRO if you have concerns or questions about the review of your application. Act quickly if you want to make suggestions or request any changes. Waiting until a couple of weeks before the review meetings is too late.
Comments
Comments are now closed for this post.